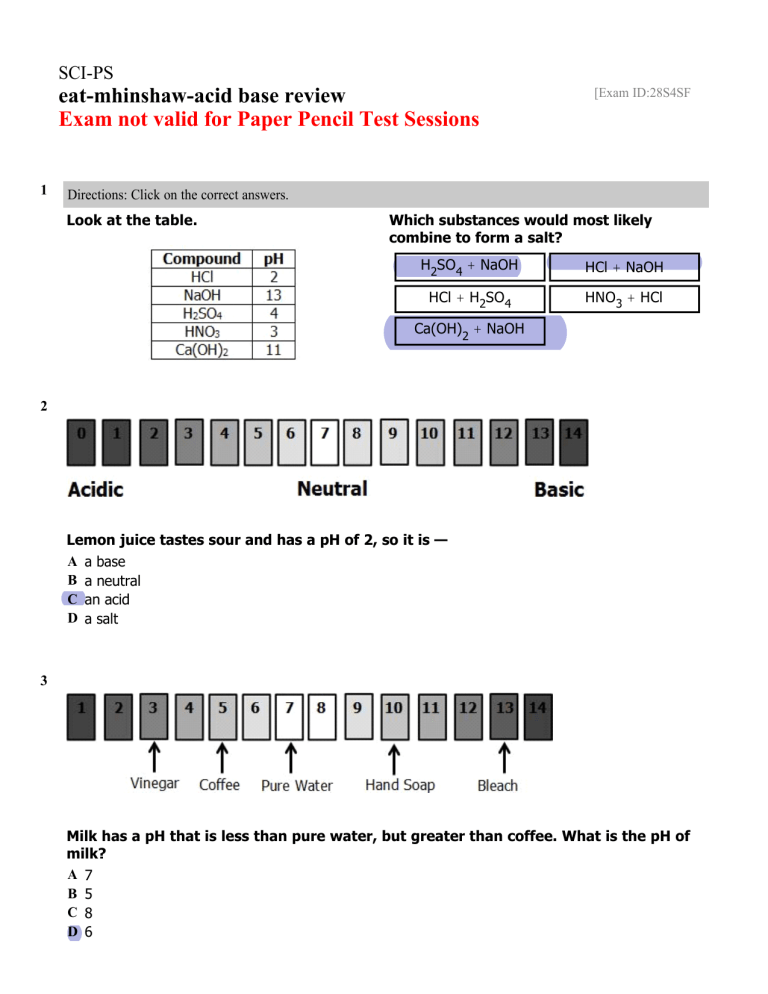
SCI-PS eat-mhinshaw-acid base review Exam not valid for Paper Pencil Test Sessions 1 [Exam ID:28S4SF Directions: Click on the correct answers. Look at the table. Which substances would most likely combine to form a salt? H2SO4 + NaOH HCl + NaOH HCl + H2SO4 HNO3 + HCl Ca(OH)2 + NaOH 2 Lemon juice tastes sour and has a pH of 2, so it is — A a base B a neutral C an acid D a salt 3 Milk has a pH that is less than pure water, but greater than coffee. What is the pH of milk? A 7 B 5 C 8 D 6 4 Which compound is organic? A NaCl B C2H2 C MgSO4 D H2O 5 Baking soda feels soapy and has a pH of 8.5, so it is — A an acid B a salt C a base D a pure substance 6 An unknown substance tastes sour and has a pH of 3. This substance must be — A an element B an acid C a salt D a base 7 Maple trees prefer soil that has a pH of 5.5 to 6.5. These trees grow best in soil that is — A slightly basic B very basic C very acidic D slightly acidic 8 When an acid reacts with a base, all of the following are produced EXCEPT— A water B a salt C a neutral substance D a strong acid 9 NaCl is classified as — A a base B a salt C an acid D a mixture 10 A student has a liquid solution with a pH of 9. The student adds vinegar to the solution lowering the pH to 5. What change occured in the solution? A The solution changed from a basic solution to an acidic solution. B The solution changed from a neutral solution to an acidic solution. C The solution changed from an acidic solution to a basic solution. D The solution changed from an acidic solution to a neutral solution. 11 A substance that produces hydroxide ions in solution is called – A A base B A neutralizer C An atom D An acid 12 Drag the terms to the correct place on the diagram. Using the terms provided; correctly identify the property described. Acid Base 13 The flowers of a hydrangea plant turn blue when the soil is acidic and pink when the soil is basic. These flowers must contain a substance that is — A a base B an indicator C an acid D a salt 14 A substance made from an acid and a base is — A a salt B an acid C a base D an element





