Weak Bases
advertisement
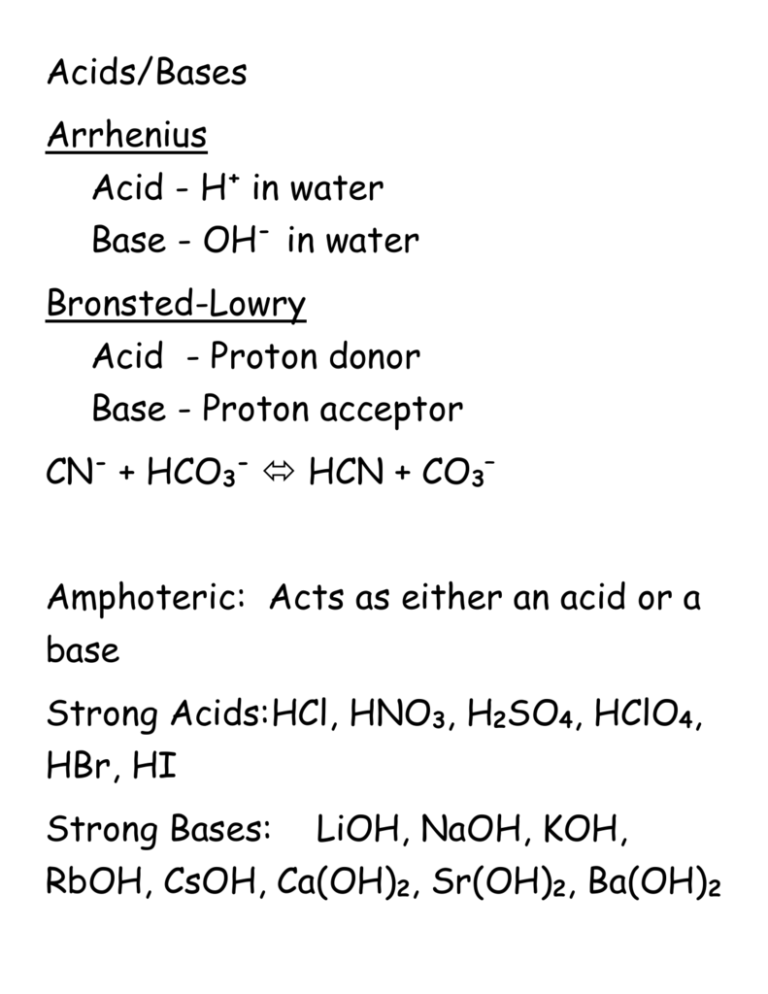
Acids/Bases Arrhenius Acid - H+ in water Base - OH- in water Bronsted-Lowry Acid - Proton donor Base - Proton acceptor CN- + HCO3- HCN + CO3– Amphoteric: Acts as either an acid or a base Strong Acids:HCl, HNO3, H2SO4, HClO4, HBr, HI Strong Bases: LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, Ba(OH)2 pH/pOH/[H+]/[OH-] pH when 0.400 g of HI is dissolved in 250 mL of water? (pH=1.90) pH when 0.065 g of Ca(OH)2 is dissolved in 700 mL of water? (pH=11.40) Weak Acids HA + H2O H3O+ + ApH of 0.10 M HC2H3O2 Ka = 1.8 x 10-5 (pH=2.88) Polyprotic acids Ka1 is much higher than Ka2 or Ka3 Weak Bases B + H2O HB+ + OHpH of 0.10M pyridine (C5H5N) Kb =1.8 x 10-9 (pH=9.13) Salts React with water in hydrolysis reaction Calculate pH of 0.10 M LiCN solution Ka (HCN) = 6.2 x 10-10 (pH=11.10) Acid/Base/Neutral NaNO3 C5H5NHClO4 KF NaNO2 NH4NO2 KCl NaNO3-N C5H5NHClO4-A KF-B NaNO2-B NH4NO2-A KCl-N Buffer: A solution that resists changes to pH when strong acids or bases are added to it. Weak acid and conjugate base Weak base and conjugate acid pH of 0.30M HC2H3O2 (Ka=1.8x10-5) and 0.40M NaC2H3O2 in 1.0L solution. (pH=4.86) Add 0.1 mole of HCl (pH=4.62) Add 0.02 mole of NaOH (pH=4.92) Titration: 50 mL of 0.2M HA (Ka = 3.5 x 10-8) with 0.25M NaOH 0mL of NaOH: pH=4.08 10 mL of NaOH: pH=6.98 20 mL of NaOH: pH=7.46 40 mL of NaOH: pH=10.25 60 mL of NaOH: pH=12.66) Ksp Solid calcium phosphate is added to water until a saturated solution is formed with [Ca2+] = 3.42 x 10-7M. Ksp? Comment on solubility. What is the solubility of a saturated solution of Cu(IO3)2 with a Ksp of 1.4x10-7M. What is the solubility of SrSO3 in 0.25M Na2SO3? (Ksp for SrSO3=3.44x10-7) Will a precipitate form when 100mL of 1.0 x 10-4M AgNO3 is mixed with 100mL of 2.5x10-4M K2CrO4? (Ksp for Ag2CrO4=1.12x10-12)











