MELTING - sced350baspinar
advertisement

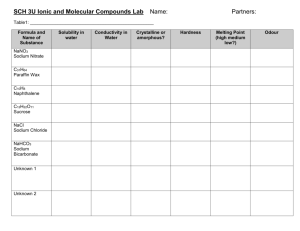
MELTING In this experiment, we saw some examples of soldi materials on the metal plate which were ice, butter, wax, table salt, menthol and vanillin. We set them on a tripod under which there was Bunsen burner.Experiment was quite simple. We turned the burner on and observed which one melted first. Of course, to make accurate observation we needed to take same amount of each substance. The melting points of the substances are as follows: m.p. of ice = 0°C m.p. of butter = 32°C m.p. of menthol = 36°C - 45°C m.p. of wax = 55°C – 65°C m.p. of vanillin = 80°C m.p. of table salt = 800°C (two different datas are those of isomers of another crystal forms.) Therefore, the order of melting is from top to bottom in the list. Melting is the phase change from solid state to liquid state. Melting1 (sometimes called fusion) is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a solid substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid 1 http://en.wikipedia.org/wiki/Melting ordering of molecular entities in the solid breaks down to a less-ordered state and the solid liquefies. An object that has melted completely is molten. The melting point of a solid2 is the temperature at which the vapor pressure of the solid and the liquid are equal. At the melting point the solid and liquid phase exist in equilibrium. As the points that became clear after the experiment; We understood the difference of intermolecular forces and molecular interaction. During the melting process, intermolecular forces between any two particles don’t change. However, since we are heating and the kinetic energy is increasing, distance between them increases a little. So, molecular interaction is weakened but intermolecular forces remain teh same. Actually, the force any two molecules never changes. Since the amount of energy given by the bunsen burner wasn’t enough to break the bonds of table salt whose melting point is extremely high compared to others, 800°C. Therefore, salt didn’t melt during the experiment. Melting is a property relevant to intermolecular forces. Particles of solids have regular arrangement, liquids and gases have random arrangement. In conclusion, in this experiment, we learnt the melting process in submacroscopic level. Temperature is increasing, so does the kinetic energy which means particles begin to move faster. Also, the force of attraction doesn’t change but the interaction is weakened due to increased distance between the particles. 2 http://en.wikipedia.org/wiki/Melting_point