ionic and covalent lab - rickert
advertisement
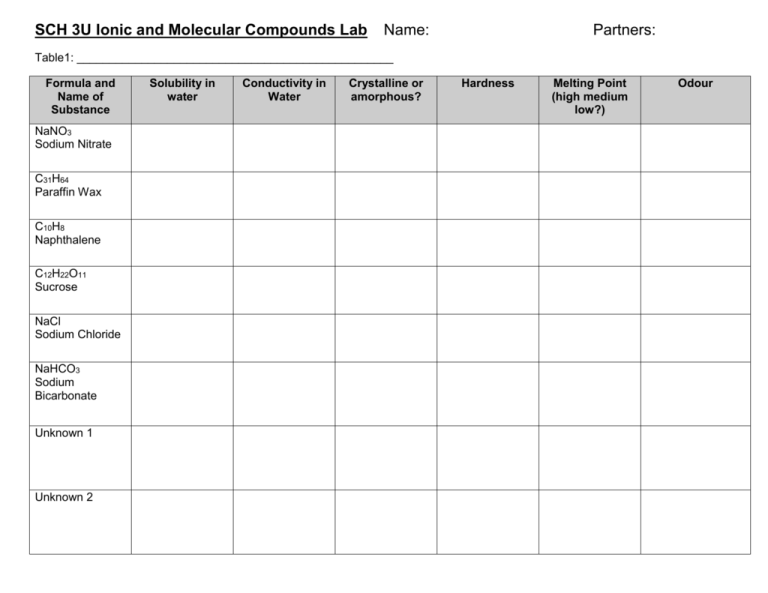
SCH 3U Ionic and Molecular Compounds Lab Name: Partners: Table1: _________________________________________________ Formula and Name of Substance NaNO3 Sodium Nitrate C31H64 Paraffin Wax C10H8 Naphthalene C12H22O11 Sucrose NaCl Sodium Chloride NaHCO3 Sodium Bicarbonate Unknown 1 Unknown 2 Solubility in water Conductivity in Water Crystalline or amorphous? Hardness Melting Point (high medium low?) Odour SCH 3U Ionic and Molecular Compounds Lab Materials: 5 small beakers Dissection Microscope Stirring rod Name: SMALL samples of six substances + 2 unknowns Electrical circuit with light bulb Partners: Mortar and Pestle Hot plate Distilled Water Tin Foil IMPORTANT: All tests for Naphthalene will ONLY be conducted in the FUME HOOD. (Do not bring it to your lab bench) Procedure: (half of the class will begin on Part A, and half on Part B, then you will switch) Obtain a small amount of each sample in a beaker and proceed to PART A or B PART A (Hardness, Solubility, Conductivity) 1) Using the mortal and pestle, grind each sample and rate its hardness on a scale of 1 – 10 (10 being the hardest). 2) Return the ground sample to the beaker, and fill the beaker half-full with water. Use the stirring rod to mix the sample with water. Record whether or not is dissolves. 3) Place two electrodes in the beaker with water and your sample to determine whether the solution conducts electricity. If it does conduct, the light bulb should light up. Wipe off the electrodes with a paper towel when you are done. PART B (Odour, Crystal Form, Melting Point) 1) Waft each sample to determine whether it has on odour. If it does, describe the odour. If it doesn’t, record that it was odourless. 2) Using the samples provided on the watch glasses, examine the structure of the sample under the dissecting scope. If the crystals have a specific shape, describe it. If there is no discernable shape, record that it is ‘amorphous’ (without form). 3) To test melting point, use the SMALLEST amount of your sample that you can see. Using a scoopula, place the sample on the tin foil on the hot plate. If it melts immediately, record that it has a LOW melting point. If it shows some signs of melting after a short time, record that it has an INTERMEDIATE melting point. If it does not melt, record that it has a HIGH melting point. PART C 1) Repeat all tests for both unknowns (this should only be done AFTER you have collected information for the 6 known substances). Answer in complete sentences on a separate sheet of paper: 1) Compare molecular and ionic compounds in terms of the properties investigated in this lab. 2) Based on your results, classify each substance as ionic or molecular. 3) Identify the 2 unknown substances and give reasons for your choices. 4) Research to answer the following questions: a) Why does sucrose dissolve in water? b) Why does sucrose form crystals? c) Why is sucrose brittle?











