MELTİNG - sced350gudu
advertisement
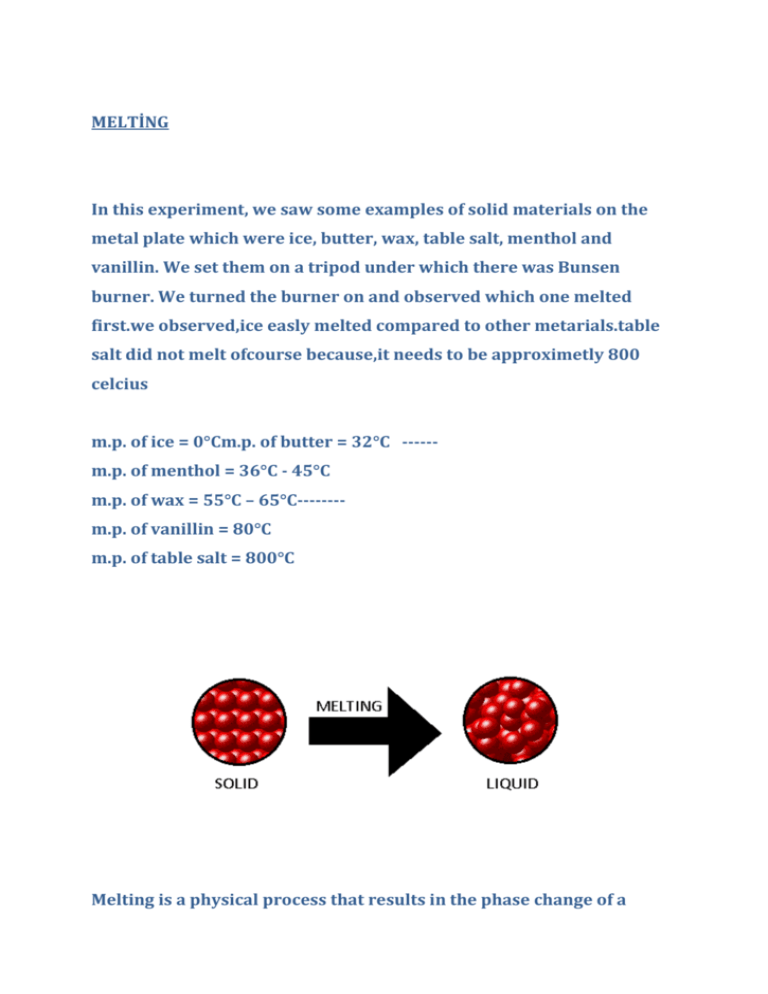
MELTİNG In this experiment, we saw some examples of solid materials on the metal plate which were ice, butter, wax, table salt, menthol and vanillin. We set them on a tripod under which there was Bunsen burner. We turned the burner on and observed which one melted first.we observed,ice easly melted compared to other metarials.table salt did not melt ofcourse because,it needs to be approximetly 800 celcius m.p. of ice = 0°Cm.p. of butter = 32°C -----m.p. of menthol = 36°C - 45°C m.p. of wax = 55°C – 65°C-------m.p. of vanillin = 80°C m.p. of table salt = 800°C Melting is a physical process that results in the phase change of a substance from a solid to a liquid. When we heat the substances, intermolecular forces are weakened .In melting process, there is a regular arrangement to random arrangement .regular arrangement represents solid when random arrangement represents liquid. After experiment we got some points; * Melting is a property relevant to intermolecular forces. * Particles of solids have regular arrangement, liquids and gases have random arrangement * intermolecular forces between any two particles do not change.Distance between particles increases so interaction is weakend.intermolecular forces remain the same