Solutions
advertisement
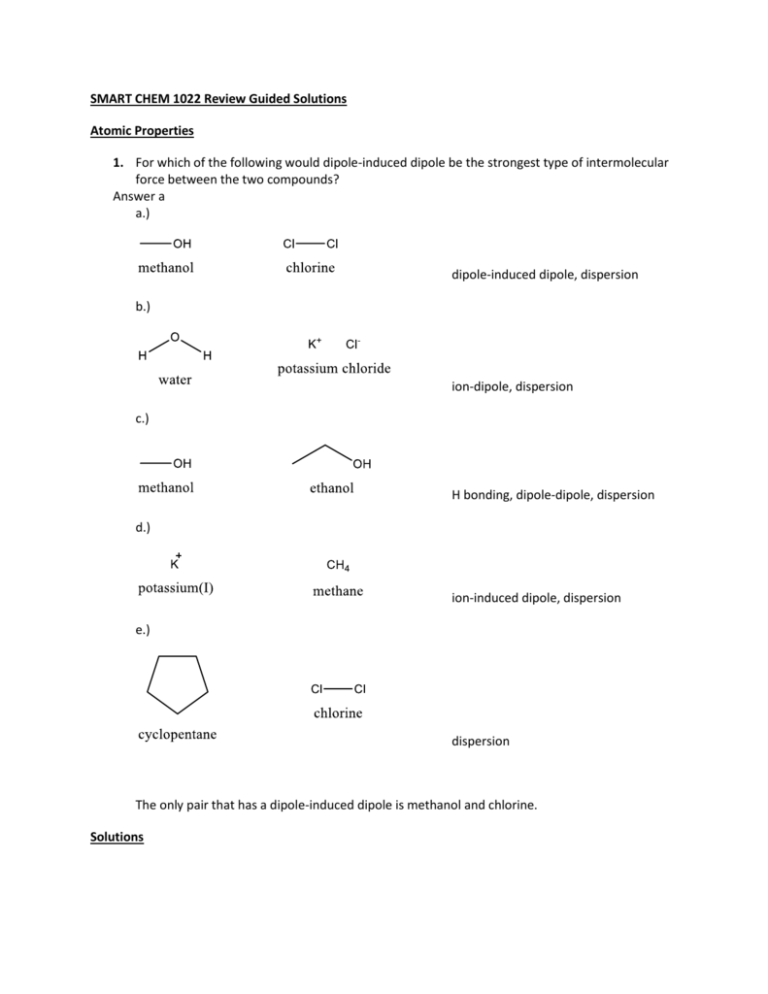
SMART CHEM 1022 Review Guided Solutions Atomic Properties 1. For which of the following would dipole-induced dipole be the strongest type of intermolecular force between the two compounds? Answer a a.) dipole-induced dipole, dispersion b.) ion-dipole, dispersion c.) H bonding, dipole-dipole, dispersion d.) ion-induced dipole, dispersion e.) dispersion The only pair that has a dipole-induced dipole is methanol and chlorine. Solutions 1. C6H12O6 is dissolved in 1.00L of H2O whose initial vapor pressure is 23.8 torr. After addition of the sugar, the vapor pressure of the solution drops to 21.4 torr. Using this information, calculate both the mole fraction of the solute and the mass, in grams, used. Using our handy-dandy periodic table, or just a love of organic chemistry, we can determine that the molar mass of C6H12O6 (or glucose) is the sum of the molar masses for each element present. This would be 12.01 for Carbon, 1.01 for Hydrogen, and 16.00 for Oxygen, so 6 ∙ 12.01 + 12 ∙ 1.01 + 6 ∙ 16.00 = 180.18 𝑔 . 𝑚𝑜𝑙 ° The handy vapor pressure equation (𝑃𝑠𝑜𝑙𝑣𝑒𝑛𝑡 = 𝑋𝑠𝑜𝑙𝑣𝑒𝑛𝑡 ∙ 𝑃𝑠𝑜𝑙𝑣𝑒𝑛𝑡 ) tells us that the vapor pressure of a solvent is equal to the product of the mole fraction of the solvent and vapor pressure of the solvent. 21.4 𝑡𝑜𝑟𝑟 = 𝑋 ∙ 23.8 𝑡𝑜𝑟𝑟. By definition, the mole fraction in this situation would be 𝑋 = 𝑚𝑜𝑙𝑒𝑠 𝑤𝑎𝑡𝑒𝑟 . 𝑚𝑜𝑙𝑒𝑠 𝑤𝑎𝑡𝑒𝑟+𝑚𝑜𝑙𝑒𝑠 𝑔𝑙𝑢𝑐𝑜𝑠𝑒 Some may be familiar with the density of water (it is 1g/ml), and from that we could determine the 23.8 moles of water. The net equation to determine moles of glucose will be: ∙ (1000𝑔 ÷ 21.4 𝑔 𝑔 18.02 ⁄𝑚𝑜𝑙 ) − 1000𝑔 ÷ 18.02 ⁄𝑚𝑜𝑙 = 𝑚𝑜𝑙𝑒𝑠 𝑔𝑙𝑢𝑐𝑜𝑠𝑒 = 6.22 𝑚𝑜𝑙𝑒𝑠. Multiplying this by the molar mass of glucose gives us the mass, in grams, or 1121.37g 2. Which of the following liquids would have the lowest boiling point? a. NaCl (0.75 m in water) b. NaN03 (0.25 m in water) c. methanol (0.25 m in water) d. pure water e. the lowest boiling point cannot be determined Answer d A solution boils at a higher temperature than a pure solvent so water has the lowest boiling point because it is the only pure liquid. 3. Assuming ideal behavior, what is the freezing point of a 0.70m solution of glucose in water? (water has Kf=1.86 °C/m, Kb=0.51°C/m, 18.0 g/mol) a. 1.30 ºC b. 0.36 ºC c. 0.00 ºC d. -0.36 ºC e. -1.30 ºC Answer e ∆Tf = K f m = Tsolvent − Tsolution The change in freezing point only depends on the concentration of the solute and the addition of solute causes freezing point depression, so ∆Tf = 0°C − Tsolution = (1.86 °C ) (0.70m) = 1.30°C m Tsolution = −1.30°C Rate Laws 1. An experiment is done to determine the rate law for a reaction which uses two reactants, A and B. Determine the rate law from the following experimental data: Initial Rate Experiment 1 2 3 4 [𝐴]0 𝑀 4.00 4.00 1.00 1.00 [𝐵]0 𝑀 4.00 2.00 2.00 1.00 𝑚𝑜𝑙 𝐿∙𝑠𝑒𝑐 640.0 80.0 20.0 2.5 My favorite way to solve these problems is to look at how changing the initial concentration affects the initial rate. Remembering that the rate for this equation should be 𝑟 = 𝑘[𝐴]𝑛0 ∙ [𝐵]𝑚 0 . If we decrease the concentrations of one of the reactants by half, then if it is first order the rate should decrease by half. If it is second order, it would decrease by ½2, and so forth. If you notice, when decreasing the concentration of B by half, we change the rate from 80 to 640, which 80 1 1 would be 640 → 8 → 23 . From this, we can see that it is like that m is 3. Keeping this in mind, by examining the change from experiment 2 to 3, while decreasing A by a fourth, the rate decreases by a fourth, suggesting that n is equal to one. Thus, the overall rate law is 𝑟 = 𝑘[𝐴]10 ∙ [𝐵]30 2. The reaction 2N205 (g) → 02 (g) + 4NO2 (g) is first order in N205. At a particular temperature, the rate constant is 1.0 x 10-4 s-1. If initially the [N205] = 0.0040 M, what is the [N205] after 20,000 seconds. a. 0.0041M b. 0.0026M c. 0.0015M d. 0.00054M e. 0.00020M Answer d [A]0 ln ( ) = kt [A]t 0.0040 ln ( ) = 1 ∗ 10−4 s −1 (20,000s) [A]t ln(0.0040) − ln[A]t = 2.0 [A]t = e−7.52 = 0.00541M 3. For a reaction in which A and B react to form C, what is the rate law for the reaction in which the following initial raw data was obtained? [A]0 (mol/L) [B]0 (mol/L) Initial Rate of Formation of C (mol/Ls) 0.2 0.4 2.00 0.2 0.8 4.00 0.2 1.6 8.00 0.4 0.4 8.00 0.8 0.4 32.00 a. rate = k [A]2[B] b. rate = k [A][B]3 c. rate = k [A]2[B]2 d. rate = k [A]2 e. rate = k [A]2[B]3 Answer a rate = k[A]a [B]b 2.00 [0.2]a [0.4]b 1b = =[ ] 4.00 [0.2]a [0.8]b 2 so b = 1 [0.4]a [0.4]1 8.00 1a = = [ ] 32.00 [0.8]a [0.4]1 2 so a = 2 Properties of solutions 1. 0.500 grams of a non electrolyte is dissolved in 25.0 mL of H2O which decreases the freezing ℃ point by 0.180⁰C. Given that the Kf and Kb for H2O is -1.86 and 0.512𝑚 respectively, what is the molar mass of the solute? First things when handling questions like this, is to figure out what role each piece of information plays in the question. For instance, the fact that it is a non-electrolyte tells us that it is a non-ionic compound and will not disassociate upon addition to water. Since the question states that the freezing point is changing, we can determine that we are only going to have to use the equation for freezing point depression, and all things related. This equation, which should be given to you on the exam, is∆𝑇 = 𝑖𝐾𝑓 𝑚. While the question gives us a Kb as well, this is a boiling point elevation constant, and irrelevant to the question, and i is a constant referring to the number of moles the solute breaks up into for every mole added to solution (i.e. KCl dissolves into K+ and Cl-, so i=2 for that). Here we do not have to worry about that. The only thing remaining is m, the molality of the solute. Using our super-funzies density of water (which is super awesome, exciting, and memorable) 1g/ml, we can get the kg of solvent and solve the equation for moles. −0.1800 C = 1 ∙ −1.86 ∙ x g 1kg 25∙1 ⁄ml∙ ⁄1000kg ∴ x = 0.00241moles. .0,500𝑔𝑟𝑎𝑚𝑠 𝑔 Using the mass provided, we can see that the molar mass is 0.00241𝑚𝑜𝑙𝑒𝑠 = 206 ⁄𝑚𝑜𝑙𝑒 Equilibrium 1. If the equilibrium for the reaction 𝐶𝑙(𝑔)2 + 2𝑂(𝑔)2 ↔ 2𝐶𝑙𝑂(𝑔)2 is 2.9 × 10−21 , what is the partial pressure of ClO2 from the reaction of 1.00 atm of O2 and Cl2? [𝐶𝑙𝑂2 ]2 2. 2 ]∙[𝑂2 ] 𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠 The definition of a equilibrium constant is 𝐾𝑒𝑞 = 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠 → 2.9 × 10−21 = [𝐶𝑙 Now we get to put all of this into another favorite tool of mine, a RICE or ICE table. This stands for Reaction, Initial, Change, and Equilibrium which is constructed as so: Reaction Initial Change Equilibrium 𝐶𝑙(𝑔)2 1.00 -x 1.00-x 2𝑂(𝑔)2 1.00 -x 1.00-x 2𝐶𝑙𝑂(𝑔)2 0 +x x [𝑥]2 This makes our equation, at equilibrium 2.9 × 10−21 = [1.00−𝑥]∙[1.00−𝑥]2 . Unfortunately, there is no other way to solve this perfectly other than to make some fundamental assumptions. Temporarily putting K in for the equilibrium, we can get the equation 𝐾 = 𝑥2 , [1.00−𝑥]3 but since K is very very small, we may assume that the concentration of products, x, is so very small, that 1x is approximately equal to 1, making our equation K=x2, and then 𝑥 = √𝐾 = √2.9 × 10−21 = 5.39 × 10−11 𝑀. For those of you interested, it is possible to solve this exactly using Newton’s Method (which you may have learned in your calculus class. Both methods do give the same answer to the 5th decimal place. 2. Calculate the equilibrium constant for the following reaction. 2A ⇌ B + 2C Initially the concentration of A was 5.5M, B was 2.0 M and no C was present. At equilibrium the concentration of A was 2.5 M. a. 6.3 b. 5.0 c. 4.6 d. 2.4 e. 1.0 K= initial concentration (mol/L) change final concentration [B][C]2 [A]2 [A] 5.5 -2x 2.5 [B] 2.0 +x 3.5 K= [3.5][3.0]2 = 5.04 [2.5]2 [C] 0 +2x 3.0 Acid Base 1. If 0.050 L of 0.100M acetic acid, with a pKa of 4.76, is titrated with 0.015L of 0.100M NaOH, what will the resultant pH be? Here is a fun little question, where we get to employ a super awesome equation known as the Henderson Hasselbalch. This one goes in a little ditty I call “puh is pika and a ha!” or 𝑝𝐻 = [𝐴− ] 𝑝𝐾𝑎 + log [𝐻𝐴]. For this equation to work we need a pKa, which is given, and the amount of acid and conjugate base. Those of you familiar with acetic acid, or vinegar, may know that it is a weak acid while sodium hydroxide is one of the strongest bases. Thus, we can assume that the base will react to completion with the acetic acid, meaning we have to use some stoicheometry. In order to do this, we need to convert both of our reactants into moles. Fortunately, the product of Molarity and volume is moles. If we assume that all of the hydroxide deprotonates the acid, then the amount of HA or acid will be given by 𝑚𝑜𝑙𝑒𝑠 𝐴𝑐𝑒𝑡𝑖𝑐 𝐴𝑐𝑖𝑑 − 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 = 𝑚𝑜𝑙𝑒𝑠 𝑟𝑒𝑚𝑎𝑖𝑛𝑖𝑛𝑔 𝐴𝑐𝑒𝑡𝑖𝑐 𝐴𝑐𝑖𝑑. When you plug all of this in to the equation it goes something like… 0.015𝐿×0.100𝑁𝑎𝑂𝐻 𝑝𝐻 = 4.76 + log 0.050𝐿×0.100𝑀 𝑎𝑐𝑒𝑡𝑖𝑐 𝑎𝑐𝑖𝑑−015𝐿×0.100𝑁𝑎𝑂𝐻 = 4.39 While this equation suggests the necessity of using concentrations, if the acid and base are in the same reaction flask, then the two concentrations would use the same volume, canceling one another out, leaving only the moles left. 2. What is the pH of 0.90 M NH3 solution? Kb=1.8x10-5 a. 2.56 b. 4.56 c. 7.40 d. 9.10 e. 11.60 NH3 (aq) + H2 0 ⇌ NH4+ (aq) + OH − (aq) Kb = [OH − ][NH4+ ] [x][x] = = 1.8 · 10−5 [NH3 ] [0.90 − x] The value of Kb is very small so we can assume that x is very small so the equation becomes[x][x] = 1.8 · 10−5 [0.90] [x] = [OH − ] = 0.004025 − log[OH − ] = 2.395 = pOH pH = 14 − pOH = 11.60 Thermodynamics 1. Under what conditions is a reaction non-spontaneous at all temperatures? Something which I highly recommend learning is that spontaneous reactions occur when the change in gibbs free energy is less than zero, meaning non-spontaneous reactions occur when the change in Gibb’s free energy is greater than zero. Setting up the equation ∆𝐺 ° = ∆𝐻 ° − 𝑇∆𝑆 ° as an inequality where ∆𝐺 ° > 0 then 0 > ∆𝐻 ° − 𝑇∆𝑆 ° Playing around with this, it is possible to see that so long as ∆𝑆 ° < 0 and ∆𝐻 ° > 0, then the reaction will be non-spontaneous. 2. For the following reaction at equilibrium, which choice gives a change that will shift the equilibrium to the left? ∆H°= 30kJ 2NOBr (g) ⇌ 2NO (g) + Br2 (g) a. b. c. d. e. increase the volume remove Br2 remove NOBr remove NO two of these changes Answer c a. An increase in volume would decrease the pressure, so equilibrium would shift to the right. b. Removal of Br2 would decrease Q so the reaction would have to shift to the right to go back to Keq. c. Removal of NOBr would increase Q so the reaction would have to shift to the left to go back to Keq. d. Removal of NO would decrease Q so the reaction would have to shift to the right to go back to Keq. 3. Which of the following results in a decrease in the entropy of the system? a. 02 (g), 300K → 02 (g), 400 K b. H20 (l) → H20 (g) c. 2N02 (g) → N204 (g) d. NH3 (s) → NH3 (l) e. 2H20 (g) → 2H2 (g) + 02 (g) Answer c a. Heat lost by the surroundings is gained by the system so there is an increase in entropy of the system. b. The entropy of the system increases as water absorbs heat and changes to gas. c. The entropy of the system decreases because the amount of gas decreases. d. The entropy of the system increases as solid NH3 absorbs heat and becomes liquid. e. The entropy of the system increases because the amount of gas increases. 4. Given N2H4 (l) + 2H20 (l) ⇌ N2 (g) + 4H20 (g) ∆°H = -530kJ at 298K Which of the following is true? a. the reaction is spontaneous at high temperatures b. the reaction is spontaneous at low temperatures c. the reaction is spontaneous at all temperatures d. the reaction is not spontaneous at any temperature Answer c The ∆H < 0 so heat is released by the system and so the total entropy will be positive and the reaction is spontaneous at all temperatures Electrochemistry 1. A galvanic cell uses a hydrogen electrode and lithium. Given that the standard reduction potential of lithium is -3.0401 and that of hydrogen is zero, what is the chemical potential of the battery if the initial concentrations of the ions are 5mM each? Looking at the periodic table, we can guess that Lithium, like Hydrogen, will be only capable of forming a plus one charge state. Thus, the total reaction is going to be 2𝐿𝑖 + 2𝐻 + → 2𝐿𝑖 + + 𝐻2 , and the standard reduction of the reaction will be 3.0401V. Using the standard reduction equation, 𝐸 = 𝐸 ° − 0.0592 log 𝑄, 𝑛 we can determine the reduction potential of the cell. Plugging in our reaction, we get something closer to being a plug ‘n chug: 3.0401 − 0.0592 [Li+ ]2 log . 𝑛 [H+ ]2 Our remaining variable, n, corresponds to the number of electrons transferred in the reaction. As a rule, it will always be the total amount of change in charge on each side, in this case it is 2. Thus, our answer is 3.0401 − 0.0592 [5×10−3 M]2 log [5×10−3 M]2 2 = 3.0401V. 2. An electrochemical cell involves the following half-reactions. Under standard conditions, what species are produced at each electrode? Ag+(aq) + e- → Ag(s) 0.80V Cu2+(aq) + 2e- →Cu(s) 0.34V a. Ag+ (aq) is produced at the cathode and Cu(s) at the anode b. Ag+ (aq) is produced at the anode and Cu (s) at the cathode c. Ag (s) is produced at the anode and Cu2+ (aq) at the cathode d. Ag (s) is produced at the cathode and Cu 2+ (aq) at the anode Answer d The spontaneous reaction that occurs is: 2Ag+ + Cu(s) → 2Ag(s) + Cu2+ Ecell = Eºcathode(reduction) - Eºanode(oxidation) = 0.80V-0.34V = 0.46V The copper is oxidized at the anode and the silver is reduced at the cathode in the overall reaction because Ecell must be positive for a spontaneous process. 3. For the reaction Zn(s) + Cu2+(aq) ⇌ Cu(s) + Zn2+(aq) the cell voltage is 1.10 V under standard conditions. What is the voltage if [Cu2+] =0.200M and [Zn2+]=0.060M? a. 1.01 V b. 1.12 V c. 1.19 V d. 1.26 V e. 1.41 V Answer b Standard concentrations are: [Zn2+] = [Cu2+]= 1 M at 298.15K where Q= Ecell [Zn]2+ [Cu]2+ J 8.314 ( ) (298.15K) RT 0.060M mol rxn ·K 0 = Ecell − lnQ = 1.10V − ln ( ) = 1.12V − mol e J nF 0.200M (2 ) (96485 ) − mol rxn V mol e











