International chemistry
advertisement

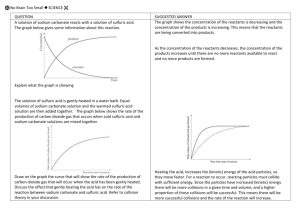
International chemistry The following passages have been taken from science textbooks. Read them carefully. Passage 1 Quand on melange de l’acide sulfurique (H2SO4) avec du cuivre carbonate (CuCO3), le cuivre carbonate commence à pétiller. Il pétille parce que l’un des produits de cette réaction est un gaz qui s’echappe. Ce gaz est du dioxide de carbone, qui est composè de carbone et d’oxygene. On note l’equation de la réaction entre l’acide sulfurique et le cuivre carbonate: H2SO4 + CuCO3 CuSO4 + CO2 + H2O A B Quel est le produit de la réaction entre MgCO3 et HCl? Quel acide réagirait avec MgCO3 pour produire MgSO4? Passage 2 Wenn verdünnte Schwefelsäure(H2SO4) Kupferkarbonat (CuCO3) zugefügt wird, beginnt sie zu schäumen. Der Grund ist, dass eines der Reaktionsprodukte ein Gas ist, welches entweicht. Dieses Gas ist Karbondioxyd, welches die Elemente Karbon und Sauerstoff enhält. Wir können die reaction zwischen Schwefelsäure und Kupferkarbonat in der folgenden Gleichung erfassen: H2SO4 + CuCO3 CuSO4 + CO2 + H2O A B Was wird in einer Reaktion zwischen MgCO3 und HCl produziert? Welche Säure produziert MgSO4 wenn sie mit MgCO3 reagiert? 1 2 3 4 What are the pages from these textbooks about? Summarise their contents in a few sentences. Answer the questions contained in the French textbook. What can you say about the answers to the questions in the German textbook? Think about these textbooks from different countries. What do they teach you about the language of science? Write a scientific sentence which describes the reaction between copper carbonate and sulphuric acid (forming copper sulphate, carbon dioxide and water) so that it could be understood by a person who does not understand or speak any English. 5 Reactions in symbols Since atoms in a reaction cannot be destroyed, when we write a symbol equation we need to make sure we have the same number of each type of atom on each side of the equation. For example, the following equation is not correct: it is unbalanced. Zn + HCl ZnCl2 + H2 The formula for zinc chloride is ZnCl2 so we cannot change that. The formula for hydrogen is H2 and we cannot change that. We can only put extra numbers in front of the formulae: Zn + 2HCl ZnCl2 + H2 The equation is now balanced. The formulae for some compounds are given in the table. Note that some atoms are always grouped together. This is shown by brackets. The small number after the brackets shows how many of these groups you have. So (PO4)2 shows that you have two phosphate (PO4) groups – a total of two phosphorus atoms and eight oxygen atoms. Name calcium chloride calcium phosphate copper carbonate copper nitrate copper sulphate ethanoic acid (also called acetic acid) hydrochloric acid 1 2 Formula Name Formula Name Formula CaCl2 magnesium carbonate MgCO3 sodium chloride NaCl Ca3(PO4)2 magnesium chloride MgCl2 sodium ethanoate CH3COONa CuCO3 magnesium nitrate Mg(NO3)2 magnesium phosphate Mg3(PO4)2 sodium hydrogencarbonate NaHCO3 Cu(NO3)2 CuSO4 magnesium sulphate MgSO4 sodium stearate CH3(CH2)16COONa CH3COOH nitric acid HNO3 sodium sulphate Na2SO4 phosphoric acid H3PO4 stearic acid CH3(CH2)16COOH sulphuric acid H2SO4 HCI What are the products of the reaction between a metal carbonate and an acid? Use the formulae in the table to help you write balanced symbol equations for the following reactions. For each reaction, give the complete word equation as well as the balanced symbol equation. a copper carbonate + sulphuric acid b magnesium carbonate + sulphuric acid c calcium carbonate + hydrochloric acid d magnesium carbonate + hydrochloric acid e copper carbonate + nitric acid f magnesium carbonate + nitric acid g sodium hydrogencarbonate + hydrochloric acid h sodium hydrogencarbonate + sulphuric acid i magnesium carbonate + phosphoric acid j sodium hydrogencarbonate + ethanoic acid k calcium carbonate + phosphoric acid l sodium hydrogencarbonate + stearic acid