Balancing Chemical Equations
advertisement

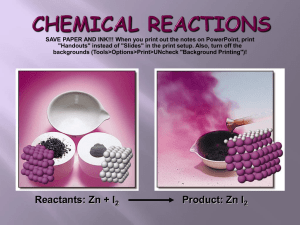
Lab book page: ____ Balancing Chemical Equations Steps for Balancing Chemical Equations: 1. Set up REP (Reactants, Elements, Products) table. 2. Put boxes around all molecules in the chemical equation. You can’t change subscripts. 3. Fill in the elements column of the REP table. 4. Fill in the number of atoms for each element in the REP table for the reactants and products assuming coefficients are 1. 5. Identify element(s) that are not balanced. 6. Identify the side (reactants or products) that needs to be balanced. It will be the lower number. 7. Find which coefficient will balance the side of the chemical reaction. Remember, the coefficient is at the front of the molecule. You can’t change subscripts. 8. Adjust the number of atoms in the REP table. 9. Repeat steps 5-8 until all atoms are balanced. In a balanced chemical equation, the number of atoms for each element of the reactants must be equal to the number of atoms for each element of the products. **Subscripts are the number of atoms of the preceding element only. ** Coefficients are the number of molecules/compounds. Examples: H2 = two hydrogen atoms 4NaCl = four molecules of Sodium Chloride giving 4 Na atoms and 4 Cl atoms 2H2 = two molecules of H giving four atoms of H Reactant + Reactant Product + Product Example: Zn + CuSO4 Cu + Symbol ZnSO4 Name What are the reactants? What are the products? Reactants Elements Products Zn Cu S O 1 Lab book page: ____ Example 1: Balance the following equations: Rewrite the equation underneath the original. 1. H2 + O2 Reactants Element Products O H H2O Example 2: Balance the following equations: Rewrite the equation underneath the original. 2. 3. 4. 5. 6. Mg + CH4 + H2CO3 O2 + KClO3 Na + O2 MgO CO2 + Ca(OH)2 CaCO3 KCl Cl2 H2 O + + H2O O2 NaCl 2