1. Hydrocarbons
advertisement

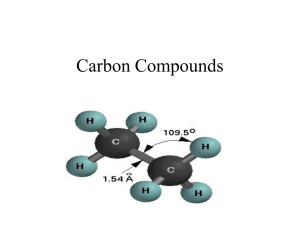
Organic and Biochemistry ! ! ! 1. Hydrocarbons Carbon atom—up to 4 bonds Hydrogen atom—forms 1 bond Molecules comprised of carbon and hydrogen Carbon and hydrogen bonds can form chains, branches, or ring structures Ex. Glucose, amino acid, octane a) Alkanes Simplest organic compounds All single C-bonds Saturated hydrocarbons Nonpolar, insoluble in water Seen with combustion reactions b) Alkenes/Alkynes One or more carbon double bonds (alkenes) Nonpolar, unsaturated due to double bonds pi (π) bonds present—increase reactivity of molecule One or more carbon triple bonds (alkynes) Aromatic Compounds Organic molecule with a ring structure 6-C based ring structure, backbone to many organic compounds Related to benzene (C6H6) Conjugated bonding system Structure with alternating double/single bonds of carbon 2. Functional Groups Atom/groups of atoms hanging off a hydrocarbon chain or ring structure Gives chemical compound its unique properties, part of compound participating in chemical reaction. Compounds with same functional group—similar chemical properties. More on Functional Groups Polar Usually contain 2 types of sites Electron-rich, Electrophiles (+ ions) attracted to this site Electron-poor, Nucleophiles (- ions) attracted to this site a) Alcohols Identified by the presence of a hydroxyl group(--OH) Hydrocarbon with an –OH hanging off Can act as a base Goes through dehydration reactions Removal of H and OH to form H2O General formula: R—OH R = hydrocarbon Ex. 1 methane --- methanol (CH3OH) Ex. 2 ethane --- ethanol (CH3CH2OH) b) Ethers General Formula: R-O-R’ Formed from alcohols 2 hydrocarbon groups linked by an oxygen c) Aldehydes General structure: RCHO Formed through alcohol oxidation/redox reaction Ex. Formaldehyde, vanilla flavor/vanillin d) Ketones Formed through alcohol oxidation General structure: RCOR, carbonyl carbon in between 2 hydrocarbons Ex. Acetone, Irone Carbonyl group is active part of aldehydes and ketones. e) Carboxylic Acids Identified by the presence of a carboxyl group (-COOH) Gives acidic properties to organic compounds Forms esters and amides Generic formula: R—COOH Ex. 1 Methanoic acid/formic acid Ex. 2 Ethanoic acid/acetic acid f) Ester Formed from alcohol and carboxylic acid molecules Water eliminated General formula: RCOOR Carboxylic acid between hydrocarbons Main reactions are hydrolysis Add water, convert back to alcohol and carboxylic acids g) Amides Formed from carboxylic and ammonia (NH3) or carboxylic acid and amine General formula: RCONH2 Main Reaction—hydrolysis Main functional group in proteins Isomers Chemical compounds with the SAME molecular formula but DIFFERENT structure/arrangement Different compounds Many molecular formulas have several different structural arrangements. Few—assign prefixes Many—use systematic naming system Homework