Carbon & Molecular Diversity
advertisement

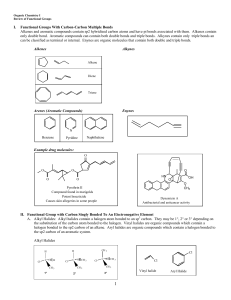
1.d.1 – There are several hypotheses about the natural origin of life on Earth, each with supporting scientific evidence (4.1). 2.a.3 – Organisms must exchange matter with the environment to grow, reproduce, and maintain organization (4.1 & 4.2). The study of carbon compounds. Usually involves the study of living things Carbon: +4 or Hydrogen: +1 Oxygen: -2 Nitrogen: -3 -4 Organic molecules made of only carbon and hydrogen. Forms 4 covalent bonds (because it has 4 valence e-) Molecular shape is tetrahedral Bonds very easily to itself Can form nearly 10 million compounds 15 known isotopes: C-14 (age of fossils), C-13, C-12 Naturally occurring in hardest (diamonds) and one of softest (graphite) forms on Earth Very small size (capable of forming multiple bonds) Major economic use—petroleum, other fossil fuels Others: pencil “lead”, steel, charcoal, medicine Compounds with the same molecular formula but have different structures. Result: Different molecular and chemical properties. No Yes Yes No 1. 2. 3. Structural Geometric (cis/trans) Enantiomers Different in covalent arrangements of their atoms. Butane Isobutane Same covalent partnership but differ in spatial arrangements. Arise from the inflexibility of double bonds. Also known as cis/trans isomers Molecules that are mirror images of each other. Usually involve an asymmetric carbon. Organisms are sensitive to even the most subtle variations in molecular architecture. This is why isomers (and their shape/characteristics) are critical to biochemistry Cells can’t distinguish between two isomers. One is an effective drug. The other causes birth defects. Used (in 1970s) as a “cure” for morning sickness A group of atoms attached to a carbon skeleton. Have consistent properties. Their number and kind give properties to the molecule. A hydrogen atom bonded to an oxygen atom. Ex. -OH Very polar. Allows the material to be hydrophilic. Forms alcohols. A carbon atom joined to an oxygen atom by a double bond. Ex. - C=O Polar tendencies Two types of Carbonyl Group compounds: • Aldehydes • Ketones A carbonyl group at the end of a carbon skeleton. Ex. - C=O H Sometimes written as - CHO A carbonyl group in the middle of a carbon chain. Ex. -C-C-C|| O Group with a carbon double bonded to an oxygen and to a hydroxyl group. Ex. - C=O | OH Written as: -COOH Also called Carboxylic Acids • Donate H+ (acid). • Form many weak organic acids. Nitrogen bonded to two hydrogens. Ex. – N-H | H Forms compounds called amines. Act as a base. IF combined with carboxyl, can act as an acid! • Ex: amino acids Sulfur bonded to a hydrogen. Ex. -SH Forms compounds called thiols. Help with protein structure. Acidic properties Phosphorus with four oxygens. Ex. -PO4 Has a net -2 charge. Sometimes written as “Pi”. Involved with energy transfers. Carbon bonded to three hydrogens. - CH3 Very non-polar and hydrophobic. Ex: fossil fuels (any other hydrocarbon) Identify what is meant by organic chemistry. Recognize the valences for the major elements of organic molecules. Recognize various types of isomers. Identify the functional groups and their structure and properties.