Lesson Plans Subject: Hon Chemistry
advertisement
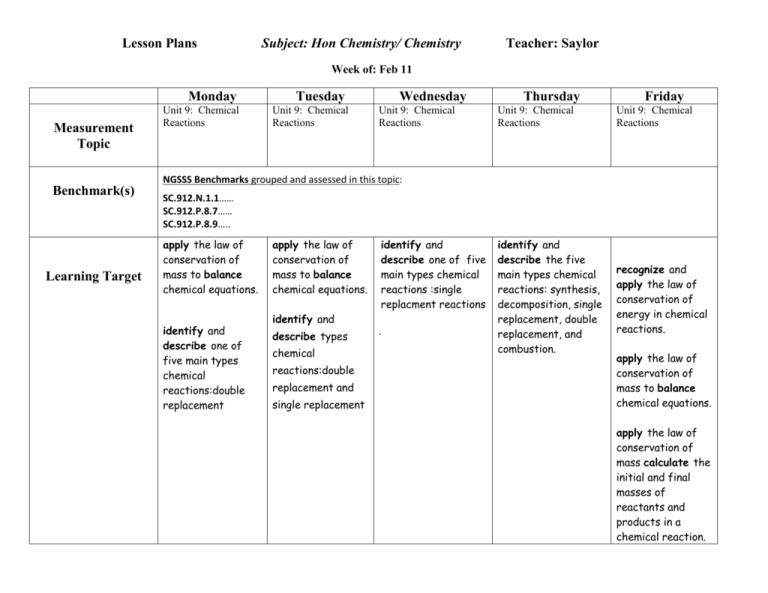
Lesson Plans Subject: Hon Chemistry/ Chemistry Teacher: Saylor Week of: Feb 11 Monday Measurement Topic Unit 9: Chemical Reactions Tuesday Unit 9: Chemical Reactions Wednesday Unit 9: Chemical Reactions Thursday Friday Unit 9: Chemical Reactions Unit 9: Chemical Reactions identify and describe the five main types chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion. recognize and apply the law of conservation of energy in chemical reactions. NGSSS Benchmarks grouped and assessed in this topic: Benchmark(s) Learning Target SC.912.N.1.1…… SC.912.P.8.7…… SC.912.P.8.9….. apply the law of conservation of mass to balance chemical equations. identify and describe one of five main types chemical reactions:double replacement apply the law of conservation of mass to balance chemical equations. identify and describe types chemical reactions:double replacement and single replacement identify and describe one of five main types chemical reactions :single replacment reactions . apply the law of conservation of mass to balance chemical equations. apply the law of conservation of mass calculate the initial and final masses of reactants and products in a chemical reaction. Lesson Students will review last week’s Nspire quiz on balancing chemical equations. A short lesson will explain double replacement reactions and how to use a solubility table to predict whether the products of the reaction are soluble (aq) or insoluble (s). Students will practice double replacement reactions ( worksheet). Assessment (D,F,S) Nspire QuizBalancing Chemical Reactions Quiz- Nspire quiz over balancing chemical reactions. Lab: Students investigate single replacement reactions experimentally. Lab Activity: Students will investigate double replacement reactions by finding a pair of solutions that do not react and a pair of solutions that do react. A word equation will be written for each reaction. Lesson: Single Replacement Reactions- Students learn to use an activity series to predict the products of a SR reaction. Worksheet practice is provided. Nspire QuizBalancing Equations Learning Targets UNIT 9A CHEMICAL REACTIONS LEARNING TARGETS Students will learn to identify synthesis, decomposition, and combustion reactions. Demonstration: Combustion reaction Demonstration: Synthesis reaction Demonstration: Decomposition reaction Practice Test: Identifying reactions, balancing equations, using chemical symbols Lab report- SR reactions Nspire Quiz- Symbols and Types of Chemical Reactions Students will apply the law of conservation of matter as they investigate a reaction involving baking soda and vinegar. Students will discuss conservation of matter via discussion of the lemonade question. Student learning will be assessed via an Nspire quiz. Unit 9 notes over symbols and reaction types will be reviewed. Nspire Quiz- Law of Conservation of Mass I will: recognize a chemical reaction as a chemical change forming new substances with new properties. list four clues that a chemical reaction has probably occurred: color change, gas formation, precipitate, temperature change. recognize and apply the law of conservation of energy in chemical reactions. apply the law of conservation of mass to balance chemical equations. apply the law of conservation of mass calculate the initial and final masses of reactants and products in a chemical reaction. interpret chemical equations in terms of reactants, products, and symbols (s, l, g, aq, →, Δ) identify and describe the five main types chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion. describe oxidation and reduction as the loss or gain of electrons. recognize which reactants are oxidized and reduced in redox reactions. identify most reactions (except double replacement) as oxidation reduction (redox) reactions.











