Reactions Unit Plan
advertisement
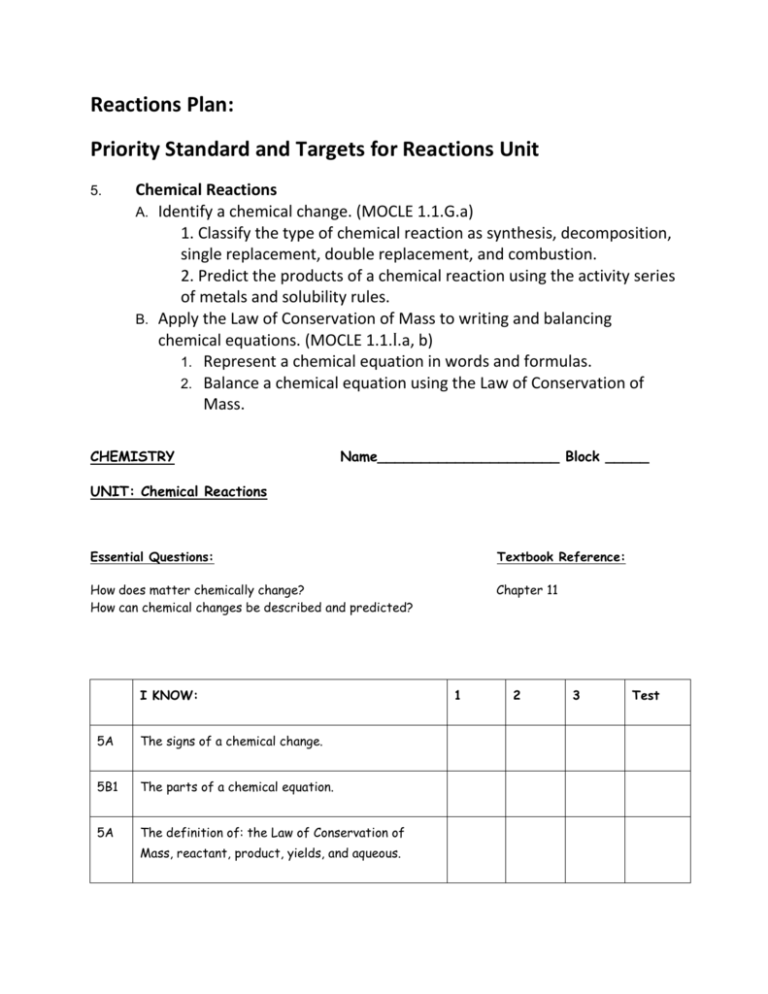
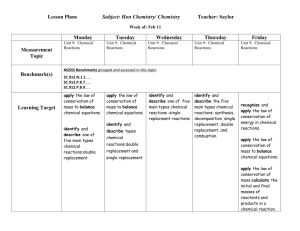
Reactions Plan: Priority Standard and Targets for Reactions Unit 5. Chemical Reactions A. Identify a chemical change. (MOCLE 1.1.G.a) 1. Classify the type of chemical reaction as synthesis, decomposition, single replacement, double replacement, and combustion. 2. Predict the products of a chemical reaction using the activity series of metals and solubility rules. B. Apply the Law of Conservation of Mass to writing and balancing chemical equations. (MOCLE 1.1.I.a, b) 1. Represent a chemical equation in words and formulas. 2. Balance a chemical equation using the Law of Conservation of Mass. CHEMISTRY Name_____________________ Block _____ UNIT: Chemical Reactions Essential Questions: Textbook Reference: How does matter chemically change? How can chemical changes be described and predicted? Chapter 11 I KNOW: 5A The signs of a chemical change. 5B1 The parts of a chemical equation. 5A The definition of: the Law of Conservation of Mass, reactant, product, yields, and aqueous. 1 2 3 Test 5B1 How to represent solid, liquid, gas, aqueous, heat, and catalyst in a chemical equation. 5B1 The diatomic elements. I UNDERSTAND: 5A2 1 2 3 Test 1 2 3 Test How to use the activity series to predict the products of single replacement reactions. 5A2 How to use the solubility rules to predict the products of double replacement reactions. 5B2 Why it is necessary to balance a chemical equation. I AM ABLE TO: 5A1 Classify a reaction as synthesis, decomposition, single replacement, double replacement or combustion. 5A2 Predict the products of a chemical reaction. Synthesis Decomp S.R. D.R. Combustion 5B1 Write a formula equation when given a word equation. 5B1 Write a word equation when given a formula equation. 5B2 Balance a chemical equation. Plan/Ideas: Steel Wool Demo Performance Event Ideas: Create a flow-chart (author Beth) [used a flow-chart in Ionic Unit Pogil.org Look at other lab files (ideas in this folder) Reactions Lab (author Lee) - excel file Assessment Ideas: White Boarding – Predicting Products, translating reactant names into formulas and predicting products, Quiz - with Smart Response or with Edline Quiz generator Progression: Complete reaction words to formulas (review of ionic naming). Balancing -“Law of Conservation of Mass” PhET online, Smart Gallery, Drill for Balancing: Demo/Lab Activities with the different type of Chemical Reactions (see files) For Absent Student or extra practice on single replacement Formative Assessments Interactive Web Pages Balancing Chemical Equations: ChemBuilder Interactive Site http://funbasedlearning.com/chemistry/ Interactive Tutorial & Practice: Balancing Chemical Equations (click on 3 links in upper left-hand corner of this site) http://www.wfu.edu/~ylwong/balanceeq/balanceq.html Animations: Interactive simulations of Single Replacement Reactions (involves activity series) http://www.chem.iastate.edu/group/Greenbowe/sections/projectfolder/flashfiles/redox/ho me.html Common Assessment District: MULTIPLE CHOICE: For the following questions, write the letter of the best answer on the line to the left. Magnesium metal reacts with hydrochloric acid solution according to the following equation: ___ Mg + ___ HCl ___ MgCl2 + ___ H2 ____ 1. What coefficients are necessary to balance the equation above? a. 1, 1 1, 1 c. 1, 1 1, 2 b. 1, 2 1, 2 d. 1, 2 1, 1 ____2. What type of chemical reaction is the reaction above? a. synthesis/ combination c. decomposition b. single replacement d. double replacement e. combustion ____3. Each substance from the reaction above should have the following states indicated as: a. (s), (l) (g), (aq) c. (s), (aq) (aq), (g) b. (s), (aq) (s), (g) d. (aq), (aq) (aq), (aq) ____4. Predict the product(s) of the following reaction: C3H8 + O2 a. C3H8O2 c. C + H2 + O2 b. CO2 + H2O d. H2 + CO2 ____5. Predict the product(s) of the following reaction: Na2CO3 + FeCl3 ? a. Na2Fe + CO3Cl3 c. Na2Cl3 + FeCO3 b. Na + CO3 + Fe + Cl d. NaCl + Fe2(CO3)3 ____6. Predict the product(s) of the following reaction: Ca + HCl ? a. b. CaCl2 + H2 CaCl2 + H c. CaH + Cl2 d. The reaction does not occur ____7. Predict the product(s) of the following reaction: NaCl + Ag ? a. b. NaClAg NaAg + Cl2 c. Na + AgCl d. The reaction does not occur ____8. Predict the product(s) of the following reaction: FeO ? a. Fe2O3 c. Fe + O2 b. Fe + O d. The reaction does not occur