Grade 10 Periodicity & Scientific Method Worksheet
advertisement
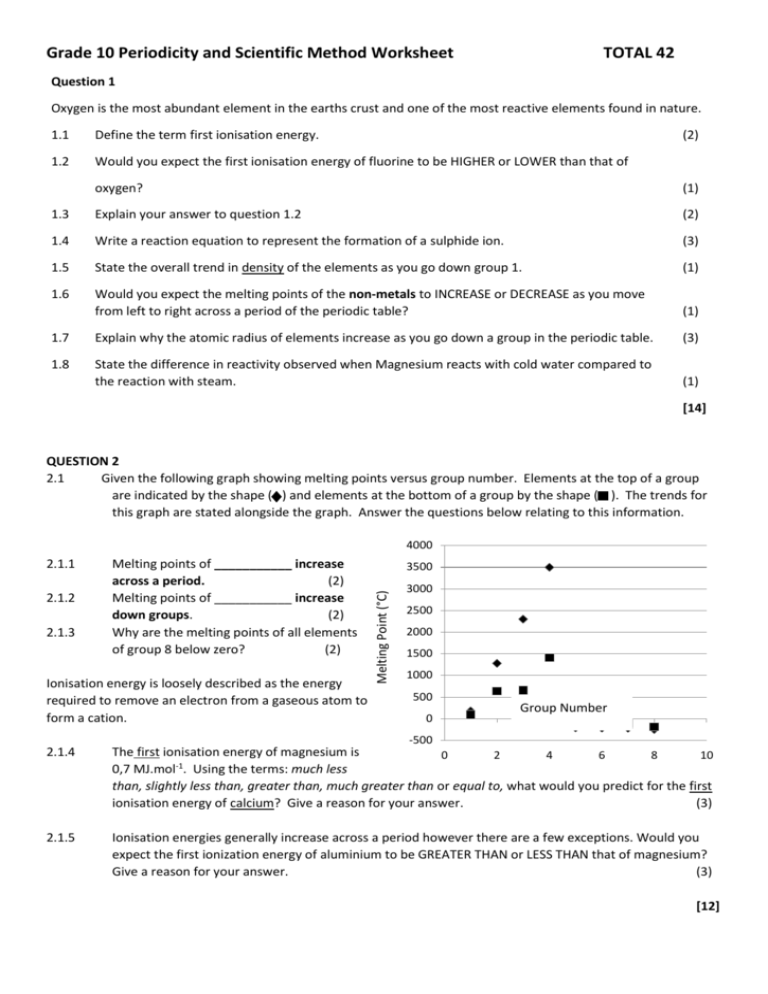
Grade 10 Periodicity and Scientific Method Worksheet TOTAL 42 Question 1 Oxygen is the most abundant element in the earths crust and one of the most reactive elements found in nature. 1.1 Define the term first ionisation energy. (2) 1.2 Would you expect the first ionisation energy of fluorine to be HIGHER or LOWER than that of oxygen? (1) 1.3 Explain your answer to question 1.2 (2) 1.4 Write a reaction equation to represent the formation of a sulphide ion. (3) 1.5 State the overall trend in density of the elements as you go down group 1. (1) 1.6 Would you expect the melting points of the non-metals to INCREASE or DECREASE as you move from left to right across a period of the periodic table? (1) 1.7 Explain why the atomic radius of elements increase as you go down a group in the periodic table. (3) 1.8 State the difference in reactivity observed when Magnesium reacts with cold water compared to the reaction with steam. (1) [14] QUESTION 2 2.1 Given the following graph showing melting points versus group number. Elements at the top of a group are indicated by the shape ( ) and elements at the bottom of a group by the shape ( ). The trends for this graph are stated alongside the graph. Answer the questions below relating to this information. 4000 2.1.2 2.1.3 Melting points of ___________ increase across a period. (2) Melting points of ___________ increase down groups. (2) Why are the melting points of all elements of group 8 below zero? (2) Ionisation energy is loosely described as the energy required to remove an electron from a gaseous atom to form a cation. 3500 Melting Point (°C) 2.1.1 3000 2500 2000 1500 1000 500 0 Group Number -500 2.1.4 The first ionisation energy of magnesium is 0 2 4 6 8 10 0,7 MJ.mol-1. Using the terms: much less than, slightly less than, greater than, much greater than or equal to, what would you predict for the first ionisation energy of calcium? Give a reason for your answer. (3) 2.1.5 Ionisation energies generally increase across a period however there are a few exceptions. Would you expect the first ionization energy of aluminium to be GREATER THAN or LESS THAN that of magnesium? Give a reason for your answer. (3) [12] QUESTION 3 3.1 Define the term “boiling point”. (2) 3.2 Is “boiling” a chemical or physical change? Give a reason for your answer. (2) 3.3 Two learners conduct an investigation. They summarize their findings in the table below. Molecular size Boiling point (oC) CH4 CH3CH3 CH3CH2CH3 CH3CH2CH2CH3 CH3CH2CH2CH2CH3 CH3CH2CH2CH2 CH2CH3 CH3CH2CH2CH2 CH2CH2CH3 CH3CH2CH2CH2 CH2CH2 CH2 CH3 -162 -88 -45 -0,5 36 69 98 126 3.3.1 Write down for this investigation the: 3.3.1 (a) Independent variable (1) (b) Dependent variable (1) (c) Control variable (Ignore environmental conditions such as pressure, temperature, volume, etc.) (1) 3.3.2 3.3.3 Write down for the learners’ investigation their: (a) Investigative question. (2) (b) Hypothesis (2) Write down three items of apparatus that the learners used in their investigation (3) 3.3.4 The learners were asked to state the conclusion for their investigation. Write down what they said. (2) [16]