File
advertisement

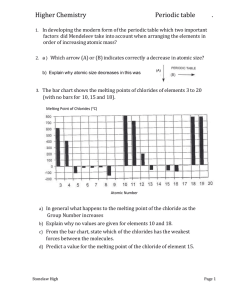
Atomic Structure – Homework 3 Part 1: Write the electronic configuration of the following using the arrow and box method: 1. C 2. Cu 3. Mg+ Write the electronic configuration of the following using the orbital method: 4. N35. Ar 6. Sc3+ 7. Mn2+ 8. Fe3+ 9. V3+ Write the electronic configuration of the following using the shorthand arrow and box method: 10. Cl11. Fe 12. Br Write the electronic configuration of the following using the shorthand orbital method: 13. Cr 14. Ga3+ 15. Pb2+ Part 2 16. Why does the first ionisation energy of atoms generally increase across a period? 17. Why is the first ionisation energy of boron less than that of beryllium? 18. Why is the first ionisation energy of oxygen less than that of nitrogen? 19. Why do first ionisation energies decrease down a group? 20. Why does helium have the highest first ionisation energy of all the elements? 21. Why is the second ionisation energy of an atom always greater than the first? 22. Why is the second ionisation energy of sodium much greater than the first? 23. Why does atomic size decrease across a period? 24. Why does atomic size increase down a group? 25. Why are cations always smaller than the corresponding atoms? 26. Why are anions always larger than the corresponding atoms? .................. Out of 26 (Grade )