Step 1
advertisement

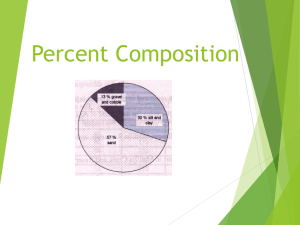
NOTES ON PERCENT COMPOSITION WHAT IS PERCENT COMPOSITION? The percent composition by mass of a compound represents the percent that each element in a compound contributes to the total mass of the compound. FORMULA: EXAMPLE 1: What is the percent composition of water (H2O)? Step 1: Figure out the molar mass from the formula 2 mol Hydrogen x 1.01g = 2.02g 1mol Oxygen x 16.00g = 16.00g 2.02 g + 16.00 g = 18.02 g/mol H2O Step 2: Divide the answer for each atom by the molar mass and multiply by 100 to get a percentage 2.02 g of H X 100% = 11.21 % H 18.02 g of H2O 16.00 g of O X 100% = 88.79 % O 18.02 g of H2O Your percentages should add up to 100% EXAMPLE 2: An 8.20g piece of magnesium combines completely with 5.40g of oxygen to form magnesium oxide. What is the percent composition of the magnesium oxide? Solution: Step 1: Find the mass of the compound 8.20g Mg + 5.40g O = 13.60 g Step 2: Find the percent by mass of each element 8.20g 13.60 g X 100% = 60.29 % Mg 5.40g 13.60 g X 100% = 39.71 % O Your percentages should add up to 100% NOTES ON PERCENT COMPOSITION WHAT IS PERCENT COMPOSITION? The percent composition by mass of a compound represents the percent that each element in a compound contributes to the total mass of the compound. FORMULA: EXAMPLE 1: What is the percent composition of water (H2O)? Step 1: Figure out the molar mass from the formula 2 mol Hydrogen x 1.01g = 2.02g 1mol Oxygen x 16.00g = 16.00g 2.02 g + 16.00 g = 18.02 g/mol H2O Step 2: Divide the answer for each atom by the molar mass and multiply by 100 to get a percentage 2.02 g of H X 100% = 11.21 % H 18.02 g of H2O 16.00 g of O 18.02 g of H2O Your percentages should add up to 100% X 100% = 88.79 % O EXAMPLE 2: An 8.20g piece of magnesium combines completely with 5.40g of oxygen to form magnesium oxide. What is the percent composition of the magnesium oxide? Solution: Step 1: Find the mass of the compound 8.20g Mg + 5.40g O = 13.60 g Step 2: Find the percent by mass of each element 8.20g 13.60 g X 100% = 60.29 % Mg 5.40g 13.60 g X 100% = 39.71 % O Your percentages should add up to 100%