Radioactivity - BHSChemistryMatt
advertisement

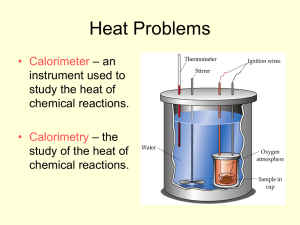
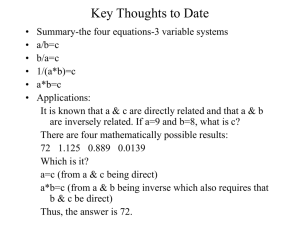
Radioactivity Review Types of Decay? Half-Life What does this mean? Create your own definition from Mine. Practice example Mercury-195 has a half life of 31 hours. If you start with 5.00g of pure mercury195, how much of it will still be present after 93 hours? What do you Know? Mercury-195 195 90Hg half life = 31 hours you have 5.00g of Hg to start with 93 hours pass What are we looking for? How much do we have left after 93 hours? Solve: 93/31= 3 half lives (time pass/half life= # of half lives) We know that the initial mass will be divided in half 3 times. first time) 5.00g/2= 2.50g Second Time) 2.50g/2= 1.25g Third Time) 1.25/2= 0.625g 0.625g will be left after 93 hours. Practice on your own: Problem 1: Gold-191 has a half -life of 12.4 hours. What mass of this isotope would remain after 49.6 hours if you started with 7.50mg sample of pure Au? Answer problem 1 49.6/12.4 = 4 half lives 1) 7.50/2 = 3.75mg 2) 3.75/2 = 1.875 mg 3) 1.875/ 2= 0.9375mg 4) 0.9375/2 = 0.46875 mg after 49.6 hr there will be 0.46875 mg left of the original sample. Problem 2 Iodine-129 with an original mass of 15.000mg loses 13.125 mg of the original mass in 39 days. What is the half life? Answer to problem 2 Original mass = 15.000 Finial mass = 15.000 - 13.125= 1.875 mg 39 days pass 1 half life = 15/2 = 7.5 2 half life = 7.5/2 =3.75 3 half life = 3.75/2= 1.875 39/3 =13days