Percentage Composition: Chemistry Calculations & Practice
advertisement

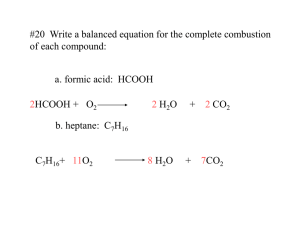
Percentage Composition What is percentage composition? The mass of each element in a compound compared to the entire mass of the compound and multiplied by 100 percent is called the percentage composition of the compound. To calculate percentage composition: Mass of element X Total mass of compound 100 = % The percentage composition of a compound tells you the percent of the mass made up by each element of the compound. This can be determined by calculating the molar mass of a given chemical formula. Calculating percentage composition of a compound: EX: What is the percentage composition of hydrogen and oxygen in H2O? Formula Mass = H= 2 atoms X 1 amu= 2 amu O= 1 atom X 16 amu= 16 amu 18amu Percentage of Hydrogen = Percentage of Oxygen = 2.0 g H2 18 g H2O X 100 = 11% 16g O 18g H20 X 100 = 89% (CHECK: Percentages should equal 100%.) Calculating mass of an element in a compound: EX: How many grams of oxygen are in 5 g of H2O? __X__ = 16g O 5 g H 20 18gH20 (Cross Multiply) 18 x = (16) (5) x = 80 18 x = 4.44 g O PRACTICE PROBLEMS KNO3 H2SO4 C2H5OH C6H5NH2 What is the percentage of carbon in CO2? How many grams of carbon are in 25 g of CO2? Find the percentage composition of a compound that contains 2.63g of carbon, 0.370g of hydrogen, and 0.580g of oxygen in a 3.58g sample of the compound.