grade 12 science organic chemistry
advertisement

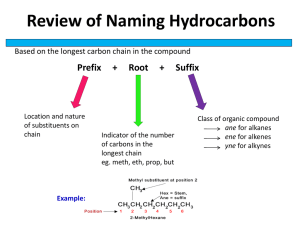
GRADE 12 SCIENCE ORGANIC CHEMISTRY Organic chemistry is the study of the compounds of carbon, but does not include carbonates hydrogen carbonates, carbides and the oxides of carbon. There are millions of organic compounds; some very simple and others complicated with large molecular masses. The reasons for the large variety of compounds are that carbon atoms: 1. can covalently bond with one another to form straight or branched chains (catenation). 2. can covalently bond with other atoms - usually non-metals. 3. can supply four electrons for four different covalent bonds. 4. Can form double, single or triple bonds Hydrocarbons – contain carbon and hydrogen ONLY Formulae of Organic Compounds Molecular formula – no of atoms of each element preset Structural formula – shows all the atoms and bonds in the molecule Condensed structural formula does not show C-C and C-H single bonds Molecular formula Structural formula Condensed structural formula H H H H H C C C C C C H H H H H H H H H H H H C H H H C C C C C H H H H OH H Functional Groups and Homologous Series Definition: A homologous series is a family of organic compounds having the same functional group but different chain lengths. A functional group is the part of the molecule that determines its chemistry. Properties of homologous series: 1. They have the same general formula (e.g. alkanes CnH2n+2). 2. Each member differs from the next by -CH2-. (e.g alkanes CH4, C2H6, C3H8…) 3. They have similar chemical properties. (e.g all alkanes burn to form CO2 and H2O) 4. Their physical properties change gradually as their molecular masses increase. The higher the mass the greater the intermolecular (van der Waal) forces. This results in increasing melting and boiling points, increased viscosity and decreasing vapour pressure. (e.g. methane (CH4) is a gas but pentane (C5H12) is a liquid) 5. They can be prepared by the same general methods. IUPAC naming of organic compounds The name has three parts Prefix Where and what other groups 1. 2. 3. Parent Number of carbon atoms Suffix Homologous series Identify the longest chain containing the main functional group (homologous series). Number carbons in the chain and give it the parent name. Identify functional groups and side chains. The prefix for the length of a chain (or side chain) is taken from the following: 1C- meth 2C- eth 3C- prop 4C- but 5C- pent 6C- hex 7C- hept 8C- oct SERIES FUNCTIONAL GROUP ALKANE C-C ETHANE C2H6 ALKENE C=C PROPENE C3H6 ALKYNE C≡C ETHYNE C2H2 ALCOHOL C-OH METHANOL CH3OH O ALDEHYDE C C H O KETONE CARBOXYLIC ACID C C C O C O H EXAMPLE Structural formula NAME (formula) PROPANAL C2H5CHO PROPANONE CH3COCH3 ETHANOIC ACID CH3COOH HALOALKANE -Cl....chloro-Br....bromo-I.......iodo- 2-BROMOPROPANE CH3CHBrCH3 ESTER O C C O C METHYL ETHANOATE CH3COOCH3 NOTE: There are 4 bonds per carbon (ALWAYS). The position of the side chain or functional group is shown with a number. The choice of number is usually such that the smallest possible numbers are used. The -an- in compounds (e.g. butanol) tells one that the C to C bonds are single bonds only. Name the following compounds: H H H H O H C C C C H H O H C H H H H 2. H H H H H H C C C C C C C H H H H H H 1. H 3. O O H3C CH2 CH2 C CH2 CH2 C CH2 H H3C 5. CH2CHCH2CHBrCH2CH3 4. H3C CH2 CH2 C O CH2 CH3 6. CH3CH2COCH2CH2CH2CH3 Complete the following table: Compound name hept-3-yne 4-bromo-2,4-dimethylhex-1-ene 3-chloropent-2-one 5,7-dimethyloctanal 2-iodohexanoic acid butylpentanoate Structural formula Condensed structural formula ISOMERS Definition: molecules with the same molecular formula but different structures. There are several types of isomerism - we only deal with STRUCTURAL ISOMERISM which has 3 sub-types: CHAIN ISOMERISM different chains Eg C4H10 Note: POSITIONAL ISOMERISM functional groups at different positions on chain eg C5H11OH FUNCTIONAL ISOMERISM different functional groups eg C3H6O Sometimes the isomer is in a different homologous series. Isomers will have different physical properties and chemical properties. Exercise: Draw the structural formula and name all the isomer bromoalkanes with the formula: C4H9Br THE ALKANES (suffix ‘-ane’) - hydrocarbons with the general formula CnH2n+2.(Note: a cycloalkane will have the formula CnH2n.) - have only C - C single bonds and are said to be saturated e.g. 2,2-dimethylpropane H H C H H H C C C H H C H H H H H We draw them in 2-D but they are actually 3-D and the carbon chain zig-zags. REACTIONS OF ALKANES: - generally unreactive COMBUSTION: - an oxidation reaction - burn in a plentiful supply of air to produce carbon dioxide and water - exothermic reaction. - in a limited air supply incomplete combustion occurs and carbon monoxide is also obtained. alkane + O2 H2O + CO2 ∆H<0 eg octane HALOGENATION: - a substitution reaction (one or more hydrogens are replaced by a functional group). - reaction conditions: X2 (X = Br, Cℓ) added to the alkane in the presence of light or heat - the halogen will substitute the hydrogen very slowly in the dark. In light the reaction is speeded up. CH4 + Cl2 CH3Cl + HCl eg. - halogenation may continue until all the hydrogens have been replaced so that a reaction between methane and chlorine will produce a mixture of CH3Cl and: CH2Cl2 CHCl3 CCl4 dichloromethane trichloromethane (chloroform) tetrachloromethane CRACKING: - Breaking longer chain alkanes that have limited uses into smaller and more useful bits - an elimination reaction (saturated compounds eliminate atoms to become unsaturated). - reaction conditions: Thermal cracking - high temperatures (450°C to 750°C) and pressures (up to 70 atm) - achieves high proportions of hydrocarbons with double bonds – alkenes Catalytic cracking - catalyst (zeolites), moderate temperature and pressure - achieves high percentage of chains with between 5 and 10 carbon atoms - hydrocarbon molecules are broken up in a fairly random way to produce mixtures of smaller hydrocarbons, some of which have carbon-carbon double bonds. eg. C15H32 2C2H4 + C3H6 + C8H18 OR C15H32 C2H4 + C4H8 + C9H20 ALKANES and INDUSTRY - Alkanes are the main constituent of crude oil. - Crude oil is largely a mixture of alkanes of varying size and shape. - Due to their varying boiling points crude oil can be separated into fractions by distillation. - The fractions have various industrial uses, but because the combustion of alkanes is so exothermic, the most important use is as a source of energy. Petrol is one such fraction. - The longer chain hydrocarbons in crude oil have limited applications whilst shorter ones are in high demand - Cracking provides the shorter alkanes used as fuels and small alkenes that are used in polymer synthesis THE ALKENES (suffix ‘-ene’) - hydrocarbons with the general formula CnH2n. - contain at least one C to C double bond and are said to be unsaturated. - if an alkene has two double bonds it is a diene. - formed by cracking alkanes or elimination (water is eliminated) reactions of alcohols (see later) REACTIONS OF ALKENES - more reactive than alkanes due to unsaturation COMBUSTION: as for alkanes if there is enough oxygen. eg. But-2-ene and oxygen HALOGENATION: - an addition reaction (a reagent adds ACROSS the double bond, breaking one of the bonds (weaker π-bond), resulting in a saturated product. - addition of X2 (X = Cℓ, Br) across the double bond of alkenes to give a dihaloalkane - reaction conditions: X2 (X = Br, Cℓ) added to alkene eg. CH2CH2 + Br2 CH2BrCH2Br - used as a test for an unsaturated compounds as the brown bromine is rapidly decolourised by alkenes HYDROHALOGENATION: - addition of HX (X = Cℓ, Br, I) to an alkene to give a haloalkane - reaction conditions: HX (X = Cℓ, Br, I) added to alkene; no water must be present eg. CH2CH2 + HBr CH3CH2Br - if the alkene is not symmetrical, the H atom attaches to the C atom already having the greater number of H atoms eg. CH3CHCH2 + HI HYDRATION: - Addition of H2O across the double bond to give an alcohol - reaction conditions: H2O in excess and a small amount of strong acid (often H3PO4) as catalyst, high temperature (ie H2O is steam) eg. CH2CH2 + H2O CH3CH2OH - if the alkene is not symmetrical, the H atom attaches to the C atom already having the greater number of H atoms. eg. CH2CHCH2CH3 + H2O HYDROGENATION: - Addition of H2 across the double bond to give an alkane - reaction conditions: alkene dissolved in a non polar solvent with catalyst (Pt, Pd or Ni), high pressure H2 atmosphere eg. CH2CH2 + H2 CH3 CH3 - important reaction in food industry as hydrogenation converts liquid vegetable oils into solid or semi-solid fats, such as those in margarine THE ALKYNES (suffix ‘-yne’) - hydrocarbons with the general formula CnH2n-2 eg. ethyne but-2-yne 1-chlorohept-3-yne REACTIONS OF ALKYNES - reactive due to unsaturation – undergoes similar addition reactions to the alkenes COMBUSTION: - Ethyne (acetylene) is used in oxy-acetylene torches. It burns at a very high temperature in a high concentration of oxygen. 2C2H2 + 5O2 4CO2 + 2H2O THE ALCOHOLS (suffix ‘-ol’) - The functional group is the hydroxyl(–O-H) - they may have one or more OH groups (-diol, -triol etc) eg. propan-1,2,3-triol (glycerol - used in soaps and medicines) - Mono-alcohols have the general formula CnH2n+1O-H. Primary Alcohols Secondary Alcohols The O-H group is attached to a The O-H group is attached to a carbon to which one other carbon to which two other carbon is attached. carbons are attached. eg. 1-butanol eg. 3-pentanol Tertiary Alcohols The O-H group is attached to a carbon to which three other carbons are attached eg. 2-methyl-2-butanol - industrial preparation - reaction between steam and alkenes (addition/hydration – see alkenes) Note – ethanol is the ONLY primary alcohol that can be prepared like this. REACTIONS OF ALCOHOLS COMBUSTION - as for alkanes eg. burning ethanol HALOGENATION: - a substitution reaction (OH group is replaced by a halogen). - the substitution proceeds readily for tertiary alcohols, but is slow and needs heating for primary and secondary alcohols - reaction conditions: Primary and Secondary Alcohols - treat with concentrated H2SO4 and solid NaBr (or KBr) - H2SO4 and NaBr react to form HBr H2SO4 + NaBr HBr + NaHSO4 - HBr reacts with the alcohol to form the bromoalkane - Overall reaction eg. CH3CH2CH2OH + H2SO4 + NaBr Tertiary Alcohols - use HBr or HCℓ at room temperature eg. C(CH3)3OH + HBr C(CH3)3Br + H2O CH3CH2CH2Br + H2O + NaHSO4 DEHYDRATION: - an elimination reaction (saturated compounds eliminate atoms to become unsaturated). - in alcohols water is eliminated to form an alkene - reaction is acid catalysed - reaction conditions: heating of alcohol with an excess of concentrated H2SO4 (or H3PO4). eg.CH3CH2OH CH2=CH2 + H2O - If more than one elimination product is possible, the major product is the one where the H atom is removed from the C atom with the least number of H atoms eg. 2-butanol will react to form mainly 2-butene (not 1-butene). THE HALOALKANES (prefix ‘halo-’) – also called ALKYL HALIDES - contain 1 or more halogen atoms: (Cl chloro-; Br bromo-; I iodo-) - as for alcohols, primary secondary and tertiary halides, depending on the degree of branching in the carbon to which the halide is attached eg. 1-bromopropane 2-iodopropanoic acid 3-bromo-3-methylpentane REACTIONS OF HALOALKANES HYDROLYSIS: - a substitution reaction - aqueous bases react with haloalkanes to produce alcohols - reaction conditions:- haloalkane dissolved in ethanol, aqueous NaOH or KOH (dilute solution) added and mixture warmed gently - (same reaction occurs without NaOH, but more slowly) eg. CH3CHBrCH3 + NaOH CH3CH2OHCH3 + NaBr DEHYDROHALOGENATION: - an elimination reaction - elimination of HX from a haloalkane to produce an alkene - reaction conditions:- heat under reflux in a concentrated solution of NaOH or KOH in pure ethanol - (NO H2O present) eg. CH3CHClCH3 + NaOH CH3CH=CH2 + NaCl + water - If more than one elimination product is possible, the major product is the one where the H atom is removed from the C atom with the least number of H atoms e.g. reaction of 2 – bromobutane with hot, ethanolic KOH: THE CARBOXYLIC ACIDS (suffix ‘oic acid’) - functional group is carboxyl (-COOH). - COOH can only be found at the end of a chain and the carbon it is on is number 1. - carboxylic acids are weak acids – low Ka values, partially ionize in water e.g. 2-iodobutanoic acid REFLUX solvent vapours condense and return to flask during heating) REACTIONS OF CARBOXYLIC ACIDS ESTERIFICATION: - a condensation reaction (two larger molecules are joined by the elimination of a smaller molecule) - an ester and water are formed in the reaction - reaction conditions:- carboxylic acid is mixed with an alcohol - a little concentrated H2SO4 is added as a catalyst - mixture warmed gently eg. CH3CH2COOH + CH3OH CH3CH2COOCH3 + H2O THE ESTERS (name ‘-yl -oate’) - formed by the reaction of an alcohol and a carboxylic acid, from which the name is derived. -yl -oate eg. Ester from the reaction between butanol and hexanoic acid is named butyl hexanoate ESTERS and INDUSTRY - widely found in nature – simple ones are often are sweet smelling and give fruit and flowers their characteristic flavours and fragrances. Food and beverage manufacturers often add them to products to enhance odour and flavor of products - smaller esters have low boiling points frequently used as solvents where fast evaporation is needed eg. glue manufacture THE KETONES (suffix ‘-one’) - contain the carbonyl (C=O) functional group, - carbonyl carbon is bonded to two other carbons R O C R 1 R and R1 are alkyl chains eg CH3 or CH2CH3 - condensed formula -CO- eg. propanone CH3COCH3 - ketones are polar solvents with low boiling points eg. propanone (common name acetone) – main ingredient in nail polish remover THE ALDEHYDES (suffix ‘-al’) - contain the carbonyl (C=O) functional group, always at the END of a carbon chain - carbonyl carbon is bonded to ONE other carbon and ONE hydrogen R O C R is an alkyl chains eg CH3 or CH2CH3 H - condensed formula -CHO eg. propanal CH3CH2CHO - more reactive than ketones … thus frequently unstable PHYSICAL PROPERTIES OF ORGANIC COMPOUNDS - physical properties depend on the strength of intermolecular forces between molecules. PHYSICAL PROPERTIES: vapour pressure - the pressure exerted by a vapour at equilibrium with its liquid in a closed system. The stronger the intermolecular forces, the lower the vapour pressure. boiling point - the temperature at which the vapour pressure of a substance equals atmospheric pressure. The stronger the intermolecular forces, the higher the boiling point. melting point - the temperature at which the solid and liquid phases of a substance are at equilibrium. The stronger the intermolecular forces, the higher the melting point. RELEVANT INTERMOLECULAR FORCES Van der Waals Forces Hydrogen bonds Dispersion/London Dipole-Dipole 10 – 40 kJ.mol-1 0.05 – 40kJ.mol-1 5 – 25kJ.mol-1 All molecules Molecules with polar functional group Molecules with hydroxyl group (-OH) PHYSICAL PROPERTIES and FUNCTIONAL GROUPS ALKANE ALKENE ALKYNE HALOALKANE ALDEHYDE KETONE non-polar molecules - London forces only contain halogens (Cl, Br, I) which vary in electronegativity – C-X bonds may be polar, so some dipole-dipole forces may be present contain the carbonyl (C=O) group which is polar due to the strongly electronegative oxygen, dipole-dipole forces present δδ+ C O ESTER δ+ C O δ- contain hydroxyl functional group (OH) so hydrogen bonding present H ALCOHOL H C H H - δ O + δ H H C O δ- H + Hδ CARBOXYLIC ACID Carboxyl (COOH) functional group allows H- bonding, but stronger H-bond than alcohols - two sites available for H-bonding on adjacent molecules Oδ H3C C O H O δ+ H δ+ C CH3 Oδ- Strength of intermolecular forces: carboxylic acid > alcohol > aldehyde/ketone > ester > haloalkane > alkane/alkene/alkyne - hydrocarbons have weak IMF, thus enter the gas phase easily and vapour pressure is HIGH. They change state readily and have LOW melting and boiling points - polar compounds have stronger IMF, and thus require more energy to change phase, so melting and boiling points that are higher than the hydrocarbons and vapour pressure is lower. - alcohols and carboxylic acids have the strongest IMF, and thus have low vapour pressure at a specific temperature. Much energy is needed for them to change phase, so melting and boiling points are high. eg. IMF Boiling Point (ᵒC) Butane (Mr 58) London only 0 Propanal (Mr 58) Dipole - Dipole 49 Propanol (Mr 60) H-bonding 97 PHYSICAL PROPERTIES and CHAIN LENGTH - longer carbon chains produce stronger intermolecular forces. (larger surface area over which vdW forces can act. - in a homologous series, melting and boiling points increase as chain length increases, and the vapour pressure decreases. PHYSICAL PROPERTIES and BRANCHED CHAINS - as chains become more branched, the molecule become less ‘linear’ in shape and less areas on the molecule are in in close enough close proximity for vdW forces to have an influence. - increased branching results in weaker intermolecular forces and lower melting and boiling points. CH3 CH2 H3C H3C CH2 CH CH3 CH2 CH3 H3C CH2 CH3 CH3 CH H3C CH3