File - Spanish Point Chemistry
advertisement

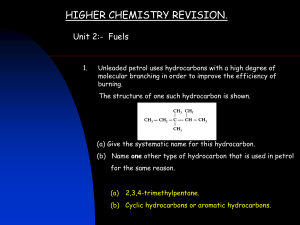
Oil refining and its Products Petrol and Crude Oil Crude oil is separated by fractional distillation works because the molecules have different boiling/condensation points many of these hydrocarbons are alkanes, and are sorted into fractions each fraction has a range of boiling points in the distillation narrow boiling ranges of limited carbon number (eg light gasoline is C5 to C7 boiling point 25C -75C) Gasoline and gas oil fractions are sources of petrol components Naptha used for high grade petrol and chemical feedstocks Fractionation of Crude Oil Fractions and their uses Refinery Gas (LPG) Light Gasoline (Petrol) Naphtha (Petrol) Kerosene (Jet Fuel) Gas Oil (Diesel Fuel) Residue Fractions (Bitumen) Natural Gas Natural gas is an extremely fuel both for domestic and industrial use. It is a mixture consisting mostly of methane, CH4, (at least 85%), ethane, C2H6, (up to 10%) and small amounts of propane, C3H8, and butane, C4H10. Liquid Petroleum Gas (LPG) The gases in the refinery gas fraction are bottled and sold for domestic use. Propane and butane from this fraction can be readily liquefied under pressure and are referred to as liquid petroleum gas (LPG). Mercaptans Very smelly, organic sulfur compounds called mercaptans are added to natural gas and LPG so that leaks can be detected Petrol Composition Complex mixture of compounds Mainly Hydrocarbons Branched – chain alkanes Aromatic Compounds Petrol in the Internal Combustion Engine Vaporised Mixed with air Compressed Ignited and burned Gases produced expand Kinetic Energy Premature Ignition Problem: Auto-ignition (i.e. knocking or pinking) Effects: a) Loss of power b) Engine damage Prevention: a) Additives b) Use suitable mixtures of high-octane compounds Octane Rating Measure of tendency to resist auto-ignite or Measure of tendency to cause knocking Low octane rating makes auto-ignition more likely Octane Rating 2,2,4-tri-methylpentane Octane Number =100 Heptane Octane Number = 0 Additives (i) (ii) Lead compounds e.g. tetra ethyl lead Prevents reactions Harmful environmental effects Phased out in 2000 Oxygenates e.g. ROR orROR1 MTBE Raise octane number Cause less pollution Mixture of compounds with high octane numbers Molecular features: Degree of branching – the more the better Chain length – the shorter the better Presence of rings – highly desirable High octane numbers can be obtained from low by: 1. Isomerisation 2. Dehydrocyclisation 3. Catalytic cracking All three methods involve the use of catalysts Isomerisation Take a straight chain alkane e.g. pentane (O.N.62) C─C─C─C─C Heat in the presence of a catalyst Chain breaks Bits rejoin to form a branched compound e.g.2methylbutane (O.N.93) C─C─C─C │ C Dehydrocyclisation Take a straight chain alkane e.g. hexane (O.N. 25) Catalyst causes change to a cycloalkane (O.N. 83) C6H14 → (CH2)6 + H2 Catalyst causes the cycloalkane to change to an aromatic compound e.g. benzene (O.N. >100) (CH2)6 → 3H2 + C6H6 Benzene Catalytic Cracking Heavy oil e.g. kerosine or diesel High temperature and catalyst Molecule breaks into several smaller molecules Unsaturated products are used as feedstock for the polymer industry Saturated products are usually high octane branched chain alkanes suitable for making petrol CH3 ─ (CH2)10 ─ CH3 ↓ CH3 CH3 │ │ CH3 ─ CH ─ CH2 ─ CH ─ CH3 + CH3 │ CH2 = C ─ CH2 ─ CH3