Chapter 17: Organic Chemistry
advertisement
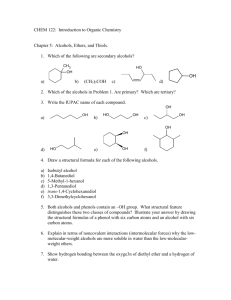
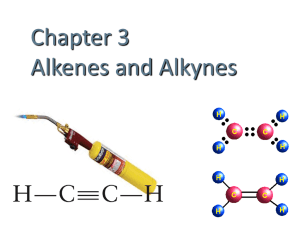
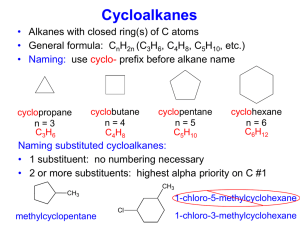
Chapter 17: Organic Chemistry Chem 1110 Figures: Basic Chemistry 3rd Ed., Timberlake and Timberlake Organic Chemistry Organic Chemistry is the chemistry of carbon containing molecules • Originally thought to be produced only by living things… organic Chemistry is the study of the composition, structure and reactions of matter • The Structure of molecules is very important in organic and biochemistry IUPAC Names: Organic Molecule Prefixes Structural Formulas Revisited Alkanes are written as structural formulas: • Revisit Chapter 6 for more details • Expanded Structure shows each bond • Condensed Structures show each carbon atom and the attached hydrogen atoms Drawing Structural (Line) Formulas Carbon atoms in a chain: • maintain their tetrahedral shape • are connected in a zigzag pattern • are drawn as two dimensional • can be written in many ways • have free rotation in straight chain Conformers • Remember, organic compounds have free rotation around sp3 carbon atoms, this leads to a variety of spatial orientations for the same structure • Conformers: different orientations of the same molecule: Conformers Let’s consider different conformers of pentane, C5H12: Isomers Isomers: • Compounds with the same molecular formula but different atomic arrangements For Example: • Butane (C4H10) has a straight chain and a branched chain form Isomers Let’s consider different isomers of pentane, C5H12: Alkanes with Substituents In the IUPAC system: • a carbon branch is named an alkyl-group • halogen atoms are named as halo-group Naming Alkanes 1. Find longest alkane chain 2. Name and number substituents 3. Substituents are numbers to give the lowest number possible 4. Substituents are named in alphabetical order, ignore prefixes di-, tri-, etc… Naming Alkanes Learning Check Provide the IUPAC name for the following: Cl CH3 | | CH3─CH2─CH─CH2─C─CH2─CH3 | Cl Drawing Alkanes from Names 2,2,3-trimethylpentane 2,4-dibromo-3-ethylhexane Take Home Draw the condensed and line structure for 2-bromo-4-chlorobutane. Classifying Carbon Atoms Primary (1°): attached to one other carbon Secondary (2°): attached to two other carbons Tertiary (3°): attached to three other carbons Quaternary (4°): attached to four other carbons Cycloalkanes Saturated compounds with a ring structure • Have a loss of free rotation • Formed by removal of a H atom form each end carbon Butane, C4H10 Cyclobutane, C4H8 Cycloalkanes Saturated compounds with a ring structure that have a loss of free rotation • Practice drawing cyclohexane, C6H12 Unsaturated Hydrocarbons Alkenes contain a carbon-carbon double bond (C = C) • Have a fixed structure around the C=C • Named for the longest carbon chain which includes the base alkene Alkynes contain a carbon-carbon triple bond (C ≡ C) • Named for the longest carbon chain which includes the base alkyne Alkenes and Alkynes cis and trans Isomers of Alkenes Alkenes have two different isomers that are determined by what groups lie on each side of the double bond: • cis: substituent groups are on the same side of the double bond • trans: substituent groups are on the opposite side of the double bond • MUST have two different groups on C cis and trans Isomers Alkynes NO rotation around the C≡C and have a straight line structure at the triple bond: • EXAMPLE: Reactions of Alkenes Addition Reactions (Combination Reactions): • Reactants add across the double bond • H2 (hydrogenation) and halogens (F2, Br2, Cl2) + 3 H2 Reactions of Alkenes Addition Reactions (combination reactions) • HF, HCl, HBr, HI + HCl • H2O, creates an alcohol + H2O Polymerization of Alkenes Polymerization is an Addition Reaction: • Addition reaction where one alkene combines with another alkene • Polymer: A molecule with a high molar mass made up of thousands of repeating units. Monomer: individual molecules used to produce the polymer. • Synthetic Polymers in Everyday Items Polymerization In polymerization, small repeating units called monomers join to form a long-chain polymer Learning Check What is the structure of polypropylene? Take Home What is the structure of polystyrene (polyphenylethene)? Recycling Plastics Recycling is simplified by using codes found on plastic items: Reactions of Alkynes • Alkynes react very similarly to alkenes but often can add two moles of reactant per mole of alkyne (due to triple bond) Aromatic Compounds Benzene (C6H6): • has six electrons shared equally among six carbon atoms in a ring • is written as two possible ring structures • is also represented as a hexagon with a circle drawn inside (common line structure shorthand) Common Aromatic Compounds in Nature and Health Learning Check Select the correct name for the following structure: 1) chlorohexane 2) chlorobenzene 3) 1-chlorobenzene Learning Check Write the IUPAC name of the following compound: CH3 Cl Take Home Draw the condensed structural formula for 1-bromo-3,4-dichlorobenzene. Alcohols and Ethers • An alcohol contains the hydroxyl (-OH) functional group • In an ether, an oxygen atom is bonded to two carbon atoms Aldehydes and Ketones Contain a carbonyl group (C=O) • In an aldehyde, the carbonyl group is attached to another carbon atom and one hydrogen atom • In a ketone, the carbonyl group attached to two other carbon atoms Oxidation Reactions In organic chemistry (and biochemistry): • Oxidation Reactions typically involve the addition of bonds to oxygen • Reduction Reactions typically involves a gain of hydrogen Classification of Alcohols Alcohols are: • classified by the number of carbon groups attached to the carbon atom bound to the -OH group: Primary Alcohol when one carbon group attached Secondary Alcohols when two carbon groups are attached Tertiary Alcohols when three carbon groups are attached Classification of Alcohols Alcohols are: • classified by the number of carbon groups attached to the carbon atom bound to the -OH group: Learning Check Classify the following as 1°, 2°, or 3° alcohols: A. CH3─CH2─CH2─OH C. OH │ CH3─C─CH2─CH3 │ CH3 OH │ B. CH3─CH─CH2─CH3 Oxidation of 1 Alcohols form Aldehydes In the oxidation [O] of a primary alcohol (1): • one H is removed from the –OH group • another H is removed from the C bonded to the –OH primary alcohol aldehyde OH │ CH3─C─H │ H O ║ CH3─C─H + HOH Ethanol (ethyl alcohol) Ethanal (acetaldehyde) Oxidation of 2 Alcohols The oxidation of 2 alcohols is similar, but a ketone is formed: secondary alcohol ketone OH │ CH3─C─CH3 │ H O ║ CH3─C─CH3 + HOH 2-propanol (isopropanol) 2-propanone (acetone) Oxidation of 3 Alcohols Tertiary (3) alcohols do not readily oxidize: Tertiary alcohol OH │ CH3─C─CH3 │ CH3 no product NO H on the C─OH to oxidize 2-Methyl-2-propanol Oxidation of Ethanol in the Body In the body: • Enzymes (proteins) in the liver oxidize ethanol • the aldehyde product impairs coordination • blood alcohol over 0.4% can be fatal CH3CH2OH Ethanol O ║ CH3CH Acetaldehyde O ║ CH3COH Acetic acid 2 CO2 + H2O Percent Blood Alcohol Concentration A breathalyzer test is used to determine blood level of ethanol Carboxylic Acids and Esters Both contain a carboxyl group, a carbonyl group attached to a hydroxyl group • A carboxylic acid contains a terminal carboxyl group • An ester contains the carboxyl group between carbon atoms • The H on the hydroxyl is replaced by the C-C bond Amines and Amides Contain a nitrogen atom or nitrogen containing group • In an amine, the functional group is a nitrogen atom • In amides, the hydroxyl group of a carboxylic acid is replaced by a nitrogen group