SOP - Webnode
advertisement

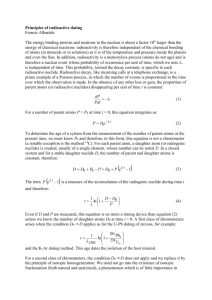
SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance Prepared By Concerned Dept 1. Checked By Concerned Dept Reviewed By QA Approved By QA-Head SOP. No. : SPL/ SOP/QC/023 Page No. : 1 of 3 Supersedes : New Revision No. : 00 Effective Date Review Date Objective To define the procedure for Operating and Calibration procedure of Analytical Balance. 2. Scope This procedure is applicable to Quality Control department at Solitaire Pharmacia Pvt. Ltd., Baddi. 3. Responsibility Executive Quality Control 4. Accountability Head Quality Control 5. Procedure 5.1. Precautions: 5.1.1. Clean the pan thoroughly prior to calibration. 5.1.2. Check that the air bubble is in the center of the level & if necessary adjust the level by turning the leveling SCREW. 5.1.3. Switch “ON” the balance & allow it to stabilize for 15 minutes. 5.2. Instrument Calibration: 5.2.1. Ensure that the balance pan is not baded and the door of the weighing chamber is close properly. 5.2.2. Press the “CAL” button, the balance will display “CAL “. 5.2.3. After few seconds the balance will display ”CAL SET” and then return to weight mode and displayed as”0000” . 5.2.4. Daily verification of the Analytical Balance: Format No.SPL/QA/001 SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance Prepared By Concerned Dept 5.2.5. Checked By Concerned Dept Reviewed By QA Approved By QA-Head SOP. No. : SPL/ SOP/QC/023 Page No. : 2 of 3 Supersedes : New Revision No. : 00 Effective Date Review Date After internal calibration is completed verify the performance of the balance by Weighing 10 mg, 50 mg, 100 mg; 1 gm and 200 gm. Record the result in Daily verification log book. 5.2.6. Calibration of measurement of uncertainty and drift of the Analytical Balance: 5.2.7. Place 200mg standard calibrated weight on the pan. 5.2.8. Record the display of the weight. 5.2.9. Remove the weight & again place the weight on the pan & record the display once again. 5.2.10. Repeat the above operation to get ten readings Calculate the standard deviation of ten readings. 5.2.11. The measurement uncertainty shall be considered satisfactory if three times of standard deviation of not less than 10 replicate weighing divided by amount weight does not exceed 0.001. Record the observations in the calibration record. 5.2.12. Now place 5.0 gm weight on the five different places as shown below to find the drift and record the observation. Format No.SPL/QA/001 SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance Prepared By Concerned Dept 6. 7. Checked By Concerned Dept Reviewed By QA Approved By QA-Head SOP. No. : SPL/ SOP/QC/023 Page No. : 3 of 3 Supersedes : New Revision No. : 00 Effective Date Records/Formats: SPL/QC/034 - Log book for daily verification of analytical balance SPL/QC/035 - Log Book for balance SPL/QC/036 - Calibration of analytical balance Annexure: NA 8. Reference: Nil 9. 10. Abbreviations: SPL - Solitaire Pharmacia Pvt. Ltd., Baddi QC - Quality Control SOP - Standard Operating Procedure Revision History: Revision No 00 Format No.SPL/QA/001 Effective date Reason for Revision New Document Review Date SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance SOP. No. Prepared By Concerned Dept Checked By Concerned Dept Reviewed By QA : SPL/ SOP/QC/023 Approved By QA-Head Log book for daily verification of analytical balance Format No : SPL/QC/034 Date 10 mg Format No.SPL/QA/001 50 mg Standards Weights 100 mg 1 gm Tolerance = ± 0.1 % 200 gm Done By Checked By SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance SOP. No. Prepared By Concerned Dept Checked By Concerned Dept Reviewed By QA : SPL/ SOP/QC/023 Approved By QA-Head Log book for Balance Format No : SPL/QC/035 Sr. No. Operation Time Date Format No.SPL/QA/001 Product Name Batch No. On Time Off Time Weight Taken Operated By Check By Remarks SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance SOP. No. Prepared By Concerned Dept Checked By Concerned Dept Reviewed By QA Approved By QA-Head Calibration of analytical balance Format No : SPL/QC/036 Date of Calibration: Next Calibration: Name of Instrument: : Analytical Balance Make : Afcoset Model No. : ------------ User Department : Quality Control Sensitivity : 0.01 mg Instrument Code : QCD/EBL/003 Calibration Schedule : Monthly Measurement of Uncertainty: Standard weight used Sr. No. : 200 mg Observation 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Mean SD Measurement of Uncertainty: = 3 X SD Weigh measured Format No.SPL/QA/001 : SPL/ SOP/QC/023 SOLITAIRE PHARMACIA PVT. LTD. RESTRICTED CIRCULATION AUTHORISED PERSONS ONLY STANDARD OPERATING PROCEDURE Title: Operation & Calibration of Analytical Balance SOP. No. Prepared By Concerned Dept Checked By Concerned Dept Reviewed By QA : SPL/ SOP/QC/023 Approved By QA-Head Drift: Standard weight used : Sr. No. 5.0 gm Observation 1. 2. 3. 4. 5. Mean RSD Remarks: The instrument is working/not working under satisfactory condition. Analyzed By: Checked By: Approved By: Date Date Date Format No.SPL/QA/001 : : :