Modulating Drug Release and Enhancing the Oral Bioavailability of
advertisement

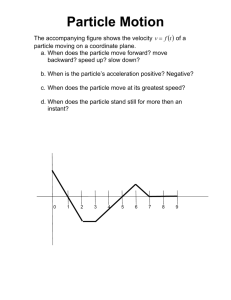
Modulating Drug Release and Enhancing the Oral Bioavailability of Torcetrapib by Solid Lipid Dispersions Yajun Liu1, Gino M. Salituro2, Keun-joong Lee2, Annette Bak1, Dennis Leung1* 1. Discovery Pharmaceutical Sciences, Merck & Co., Inc., 126 E. Lincoln Ave., Rahway, New Jersey, USA, 07065; 2. Pharmacokinetics, Pharmacodynamics and Drug Metabolism, Merck & Co., Inc., 126 E. Lincoln Ave., Rahway, New Jersey, USA, 07065 * Corresponding author: Email address: dennis_leung@merck.com Tel.: 1-732-594-6982 Supplemental Information 1. Screening stabilizers for producing solid lipid nanoparticles (SLNs) via acoustic milling Around 2 mg of solid lipid microparticles (SLMs) was weighed and added to each well of 96 well plate together with 800 mg of zirconia YTZTM milling beads (500 µm, Tosoh Corp., Tokyo, Japan). 146 µL of five surfactant solutions (Pluronic F127, Pluronic F68, Cremophor EL, Tween 80 and Tween 20) was added to each well at the concentration of giving ratio of SLMs/surfactant at 1: 0.25, 1: 0.5, 1:1, and 1:2 (w/w), respectively. The well plate was thermally sealed and then placed on LabRAM mixer (Resodyn Acoustic Mixers, Inc., Butte, MT, USA) and agitated at 40% of intensity for 1 hour. At the end of the experiment, 5 µL of slurry was withdrawn and diluted with 35 µl of water, which was subject to particle size analysis by dynamic light scattering (DynaPro Plate Reader, Wyatt Technology Corp., Santa Barbara, CA, USA). 600 1: 0.25 1:0.5 1:1 1:2 Particle size (nm) 500 400 300 200 100 0 27 c F1 oni Plur n ic o Plur F68 r EL n 80 n 20 pho wee w ee o T T m Cre Figure S1. Effect of surfactant type and ratio of particles to surfactant (w/w) on the particle size of SLNs produced from acoustic milling. 2. Particle size analysis of SLMs dissolution medium The study was conducted using dissolution testing apparatus (Distek Model 2100c, Distek, Inc., North Brunswick, NJ, USA) at 37 °C. Around 12 mg of torcetrapib-loaded SLMs precipitated at 45°C was added to 50 mL of phosphate buffer (25mM, pH 6.8) containing 0.17% of sodium dodecyl sulfate (SDS). The dissolution medium was stirred with a paddle at 50 rpm. After 1h, 3h, 6 h and 24 h, 80 µl of dissolution medium was withdrawn and analyzed for particle size using dynamic light scattering. 700 600 Particle Size (nm) 500 400 300 200 100 0 1 3 6 24 Dissolution time (h) Figure S2. Particle size kinetics of dissolution medium containing torcetrapib-loaded SLMs at 37 °C 3. Particle size analysis of torcetrapib-polymer dispersion The particle size of the spray dried amorphous solid dispersion of torcetrapib was measured by laser diffraction (SYMPATEC, Sympatec Inc., Pennington, NJ, USA). The instrument is equipped with a dispensing unit RODOS/M, a vibratory feeder VIBRI, and a laser diffraction sensor HELOS. Dry powders were measured directly with a dispersing pressure of 1.0 bar and a laser measuring range of 0.5 µm to 350 µm. A D50 particle size of 19.13 microns for the solid material was determined.