Practice Problem 1405 chapter 6.doc
1405 - Chapter 6
Language of Chemistry
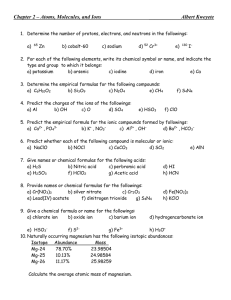
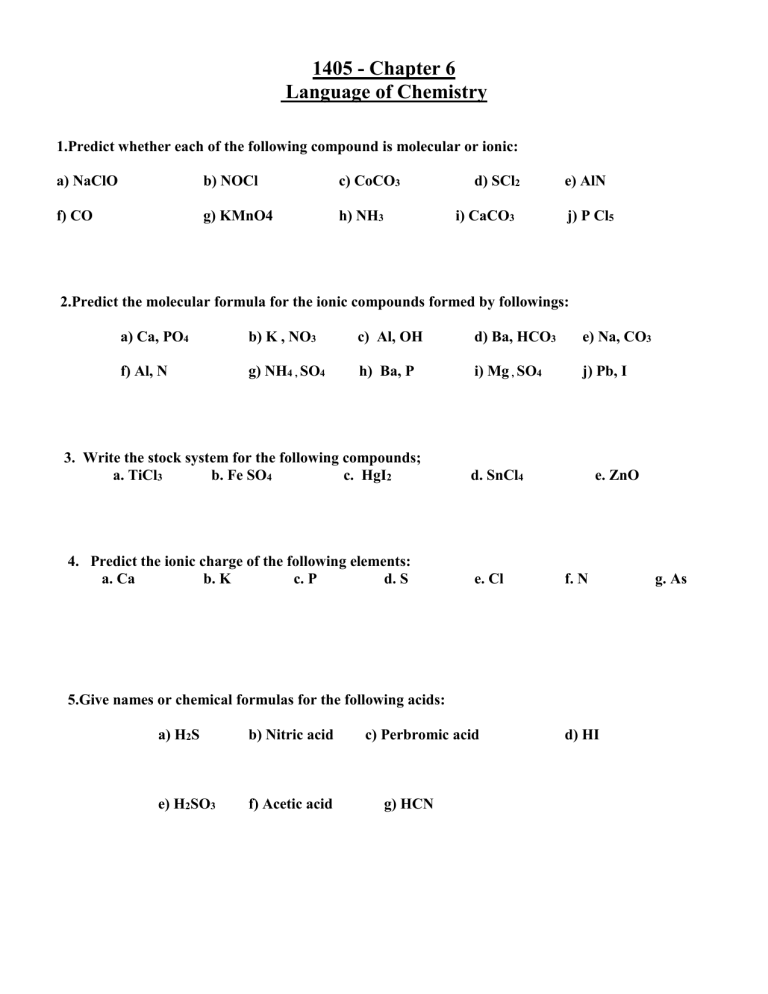
1.Predict whether each of the following compound is molecular or ionic: a) NaClO f) CO b) NOCl
g) KMnO4 c) CoCO h) NH
3
3 d) SCl
i) CaCO
3
2 e) AlN
j) P Cl
5
2.Predict the molecular formula for the ionic compounds formed by followings:
a) Ca, PO
4
f) Al, N b) K , NO g) NH
4 ,
3
SO
c) Al, OH
4 h) Ba, P d) Ba, HCO i) Mg
,
SO
4
3
e) Na, CO j) Pb, I
3
3. Write the stock system for the following compounds;
a. TiCl
3
b. Fe SO
4
c. HgI
2
d. SnCl
4
e. ZnO
4. Predict the ionic charge of the following elements: b. K c. P d. S a. Ca e. Cl f. N
5.Give names or chemical formulas for the following acids: b) Nitric acid c) Perbromic acid a) H
2
S e) H
2
SO
3 f) Acetic acid g) HCN d) HI g. As
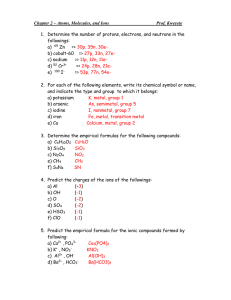
6. Provide names or chemical formulas for the followings: a) Cr(NO
3
)
3 b) silver nitrate c) Cr
2
O
3 e) Lead(IV) acetate
KClO f) dinitrogen trioxide g) S
7. Write the systematic names of the following compounds:
a. N
2
O
3
b. SO
2 c. N
2
O
5 d. P
2
O
5 e. ClO
2
4
N
4 d) Fe(NO
h) f. NI
3
2
)
2
8. Find the formula of the covalent compounds formed from the following elements. c. C and H d. P and O e. H and S a. N and I b. C and Cl
9.
Give the chemical formula or name of the followings:
a) chlorate ion b) oxide ion
d) hydrogen carbonate ion e) Hg 2+ f) S 2c) barium ion g) Fe 3+ h) H
3
O +
10. What is the difference between hydroacid and oxyacid? Give two examples of each.
11. What is the name of the following acids?
a) HClO b) HClO
2 c) HClO
3
d) HClO
4