Photosynthesis, Metabolism, Oxygen, and Carbon Dioxide (gr. 5-8)

Photosynthesis, Metabolism, Oxygen, and Carbon Dioxide
A Yakima WATERS “5-E” Inquiry Lesson
Introduction
To fully understand the co-dependent relationship between plants, animals, and the environment we will discuss and experiment with a controlled environment in class. This is a small environment in a beaker, which will contain a plant floating in a solution of pH indicator and water. The controlled variables will be the plants which will all be the same, the amount of water and pH indicator, the size of the beaker, the amount of light the system will get, and the length of time of the experiment. The measured variable will be pH, which will be measured qualitatively by using the indicator. The changed variable will be the input of gas from animals (the students) and the plants. The goal is to illustrate the gas exchange between plants and animals and the affects of gas on water quality and marine organisms.
This lesson is targeted to 6 th grade. The lesson is expected to take one 90 minute period and one 70 minute period.
Standards
6-8 SYSA Systems & Sub-systems: Any system may be thought of as containing subsystems and as being a subsystem of a larger system.
6-8 SYSB System Boundaries: The boundaries of a system can be drawn differently depending on the features of the system being investigated, the size of the system, and the purpose of the investigation.
6-8 SYSC Outputs & Inputs: The output of one system can become the input of another system.
6-8 SYSF Societal Issue: The natural and designed world is complex; it is too large and complicated to
investigate and comprehend all at once. Scientists and students learn to define small portions for the convenience of investigation. The units of investigation can be referred to as ―systems.
‖
6-8 LS2D Changes in an ecosystem: Ecosystems are continuously changing. Causes of these changes include nonliving factors such as the amount of light, range of temperatures, and availability of water, as well as living factors such as the disappearance of different species through disease, predation, habitat destruction and overuse of resources or the introduction of new species.
Standards Justification
Students should be able to identify the parts of the system they create, the bigger system that their system is a part of (i.e. the Yakima River watershed), and the subsystems within their system (i.e. plants are a living system carrying out photosynthesis and bodies of water are systems that interact with the atmosphere and biosphere) which will address the 6-8 SYSA science standard. Students should be able to identify the boundaries of the system that are appropriate for our study (i.e. to study water quality effects on plants our system should include the hydrosphere, atmosphere and biosphere) addressing 6-8
SYSB science standard. Students should be able to identify outputs of matter from one system that become inputs in another system, which will address the 6-8 SYSC science standards. Given a complex
1
societal issue of global warming, students should be able to describe the issue from a systems point of view, highlighting how changes in one part of the system are likely to influence other parts of the system
(6-8 SYSF).
Outcomes
Knowledge: Students should be able to describe the gas exchange between animals and plants.
Students should be able predict how pH indicators work. Students should also be able to explain how animal respiration/carbon dioxide production and photosynthesis/oxygen production affect the pH of water.
Skill: Design their own experiment
Use indicators to observe chemical changes
Materials and Equipment
Bromothymol blue (~4 drops for every 50mL of water)
2 Containers (such as beakers) per group
1 Straw per group
~3 Gallons of Water
1 Elodea plant per group
1 Dropper per group
1 Shell/coral per group
1 Roll of paraffin
Prior Knowledge
Students have been investigating the presence of contamination and pollution in the Yakima River watershed and looking for effects of contamination on organisms. It is assumed students understand that water contains dissolved oxygen and carbon dioxide. Students should already understand that plants need carbon dioxide, water and light to survive and grow. It is assumed that students understand that animals, such as humans, need oxygen water and food to survive. Students should have a prior understanding of the co-dependent relationship between plants and animals.
Safety
Since Bromothymol blue is a weak acid that stains clothing students will need to be cautious when handling the chemical. This chemical should not be swallowed so for young students or if you see necessary have students add carbonated water to the solution instead of blowing into it.
Engage (15 min)
Demonstrate:
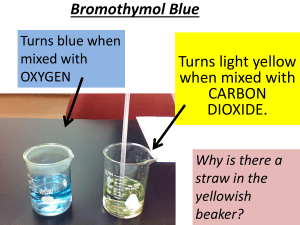
In front of the class blow into a solution of water and BTB indicator. Be sure to blow slowly and stop blowing as soon as you see the color start to change because BTB is sensitive.
Point out that simply exhaling into the dyed water changes the color.
Ask students why the color changed. A good leading question is, What gas do humans and all animals exhale?
2
Students will say: Carbon Dioxide.
Tell students that carbon dioxide enters the water when blown through the straw.
Explain to students that color change is due to the water becoming more acidic (decrease in pH) as CO₂ gas is added to the system (CO₂ gas in water forms carbonic acid which makes the water more acidic- this amount of detail is not necessary for students to know they can simply be told that CO₂ gas makes water more acidic).
By blowing into the water we are inputting carbon dioxide gas. Water can uptake gases from its surroundings and in this case the water is able to uptake the carbon dioxide we are exhaling.
Fellow/Teacher should ask: What would happen if we input (exhale) carbon dioxide into the atmosphere? Would water be able to uptake carbon dioxide from the atmosphere?
Students may say yes, no or I don’t know.
Fellow/Teacher should say: Gases like to spread themselves out and get as far away from other gases as possible. Because the atmosphere and water are in contact gases can move out of the atmosphere and into water (figure 1 can be used to illustrate this- either in a hand out or drawn on the board).
Fellow/Teacher should go on to say: Within a plant system carbon dioxide is input and oxygen is output.
As shown on figure 1 the oxygen output from plants becomes an input in the animal system and in the atmosphere system. As we have studied before water can contain oxygen which it gets from the atmosphere and plants. Just as too much oxygen can be harmful to aquatic organisms too much carbon dioxide can be harmful to aquatic organisms. High levels of CO₂ can cause water to become acidic and dissolve the shells of marine organisms (figure2).
Plants and animals are constantly exchanging matter in the form of gases. This exchange takes place through the atmosphere and through the hydrosphere.
Explore (50 min)
The goal of this lesson is to investigate how the water chemistry is affected by carbon dioxide
acidification and photosynthetic oxygen production. Leading questions:
1)
2)
3)
4)
5)
6)
7)
Why did the Bromothymol blue change the color of the water?
Why did the color of the solution change when you blew bubbles in it?
Why did the color change again in the presence of the elodea plant?
What is our relationship (animals) with plants? Why are we interdependent?
What other dependency do we as animals have on plants?
How do the lab results relate to watershed management?
What other experiments can be devised using BTB?
Begin by having the students construct environments in groups of two to four.
1) Students should be able to design an experiment using a solution of indicator and water.
They can choose to have one elodea plant and/or one shell/coral.
3
2)
3)
4)
5)
Students should be instructed to write out the variables that will be controlled, changed and measured before collecting their supplies.
Students should be able to form a hypothesis based on what they know about photosynthesis, BTB, carbon dioxide, and oxygen.
Once the students have written out all the variables and the hypothesis in their science notebooks they should show it to the fellow or the teacher and then get their supplies.
The lab experiment should be covered with paraffin and stored overnight. Any observed changes the following day should be recorded.
Using Teaching computer station with a document camera projector show an example of how to create an entry in their science notebooks that contains a hypothesis, variables, observations, and explanations.
Explain (20 min)
As students are making their observations, ask questions relating to how they approached the assignment. Make sure students explain in their own words what may have caused the changes they observed in the environment. If students need assistance, ask leading questions to help them understand the concepts of the lesson fully.
Fellow/Teacher should circulate around the room and ask students the following questions:
What types of organisms release gases?
How do gases move through the environment?
Where do organisms get carbon dioxide and oxygen from?
The goal here is to lead students to understanding why pH changes in the presence of plants and why shells dissolve in acidic water.
Extend/Elaborate (15 min)
Have a large group discussion about everyone’s data.
Fellow/Teacher says: Who would like to share their findings with the class?
Students raise hands.
Fellow/Teacher calls on students giving several students the opportunity to share with the class.
Fellow/Teacher says: Class, does everyone have the same finding? Raise your hand if you can tell me why groups agree or disagree?
Fellow/Teacher calls on one student and allows them to explain.
Fellow/Teacher says: How does everyone feel about their initial hypotheses now that they have made some observations? Raise your hand if you accept your hypothesis. Can one person who accepts their hypothesis explain why?
4
Fellow/Teacher calls on one student.
Fellow/Teacher says: Now raise your hand if you reject your hypothesis. Can one person who rejects their hypothesis explain why?
Fellow/Teacher calls on one student.
Fellow/Teacher says: Is there anything else that humans do to affect carbon dioxide levels in the system?
Students say: Yes. (Students are aware of the burning of fossil fuels by humans)
Fellow/Teacher says: The release of carbon dioxide from the burning of fossil fuels affects this system. If carbon dioxide increases in the water-atmosphere system how might that affect aquatic organisms?
Student says: Too much carbon dioxide can harm aquatic organisms. (Shown in this experiment)
Evaluate (0 min, included with Explanation Section)
While students are explaining their experimental design, observations and conclusions they will be graded using the rubric below. Students will also need to hand in their science notebooks for further grading outside of class. This will allow the teacher to see how each student understands the assignment and score their progress based on what they have been able to accomplish during the explore period. If it appears students have not had time to complete the assignment, more time will be allotted the following day or the rubric will be modified to better reflect what students had time to accomplish.
Performance Rubric
CATEGORY 4 3 2 1
Knowledge:
Experimental
Hypothesis
Hypothesized relationship between the variables and the predicted results is clear and reasonable based on what has been studied.
Hypothesized relationship between the variables and the predicted results is reasonable based on general knowledge and observations.
Hypothesized relationship between the variables and the predicted results has been stated, but appears to be based on flawed logic.
No hypothesis has been stated.
Skill:
Experimental
Design
Experimental design is a wellconstructed test of the stated hypothesis.
Experimental design is adequate to test the hypothesis, but leaves some unanswered questions.
Experimental design is relevant to the hypothesis, but is not a complete test.
Experimental design is not relevant to the hypothesis.
5
Knowledge:
Conclusion
Knowledge:
Scientific
Concepts
Conclusion includes whether the findings supported the hypothesis, possible sources of error, and what was learned from the experiment.
Conclusion includes whether the findings supported the hypothesis and what was learned from the experiment.
Conclusion includes what was learned from the experiment.
No conclusion was included in the report OR shows little effort and reflection.
Answers illustrate an accurate and thorough understanding of scientific concepts underlying the lab.
Answers illustrate an accurate understanding of most scientific concepts underlying the lab.
Answers illustrate a limited understanding of scientific concepts underlying the lab.
Answers illustrate inaccurate understanding of scientific concepts underlying the lab.
Teacher Background Info
Figure 3 below illustrates the colors that can be expected from the BTB indicator and the pH they correspond to. Plants will reduce CO₂ in the system, which will make the water less acidic and should change the color to blue. Exhaling into the system (animal influence) will put CO₂ in the system making the water more acidic and the color should change to yellow. Students should design their own experiments. Some direction may be required to assist students in experimental designs. In addition to science standards noted above, this lesson addresses:
Use of the scientific method
Listening and observation skills in order to be able to complete the tasks in the required time
The website link below may have some useful information. http://www.ehow.com/how_5812753_use-bromothymol-blue.html
By: Renee Holt, Fall 2011, for Selah Intermediate School
6
http://www.arthursclipart.org/nature/nature/page_02.htm
Figure 1: Red arrows are movement of oxygen and blue arrows are movement of carbon dioxide.
Figure 2: Ocean acidification dissolves shells and coral as shown above. A simplified version of this can be drawn on the board for students during the explain section to help them understand the changes they observe.
7
http://team8blue2010.wikispaces.com/mp4p1+Group+1
Figure 3: Color of bromothymol blue and corresponding pH. As you can see BTB is sensitive to small changes in pH which is why it is good for this type of experiment.
8