Organic Chemistry
advertisement

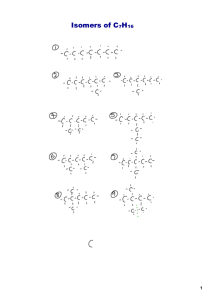
Organic Chemistry I. Carbon A. Organic Compounds = contain carbon – Exceptions: oxides and carbonates B. Carbon has 4 valence electrons, and therefore forms 4 covalent bonds – 4 single bonds: – At least 1 double bond: – 1 triple bond with 1 single bond: C. Bond Length and Strength in Organic Compounds 1. Single bonds are longest, double bonds are shorter than single, and triple bonds are shortest 2. Single bonds are weakest, double bonds are stronger than single, and triple bonds are strongest II. Hydrocarbons A. Hydrocarbon compounds contain only carbon and hydrogen 1. Saturated hydrocarbons - all carbon-carbon bonds are single bonds (allows for maximum # of hydrogens) 2. Unsaturated hydrocarbons - containing carboncarbon multiple (double/triple) bonds (minimizes the # of hydrogens B. Types of Hydrocarbons 1. Alkanes – saturated; contain only single C-C bonds Chemical Formula: Structural Formula: 2. Alkenes – unsaturated; contain at least 1 double bond between adjacent carbon atoms Chemical Formula: Structural Formula: 3. Alkynes – unsaturated; contain at least 1 triple bond between adjacent carbon atoms Chemical Formula: Structural Formula: III. Alkanes A. Isomers of Alkanes 1. Structural Isomers - Same formula, but the atoms are bonded together in a different order • Alkanes can form straight or bent chains (unbranched chains) • Alkanes can also form branched chains C4H10 Butane C4H10 2-methylpropane B. Formulas for Alkanes C. Naming Alkanes Prefix MethEthPropButPentHexHeptOctNonDec- # of Carbons 1 2 3 4 5 6 7 8 9 10 Suffix -ane Type of bond single bonds C. Naming Alkanes (cont) 1. Other rules: • For branched hydrocarbons use the longest continuous chain for the root name. • Alkanes missing one H atom can have another hydrocarbon attached at the missing H point. • Specify the names of substituents by numbering the C atoms starting at the end closest to the branching. • If a substituent occurs more than once use a prefix to show this. CH3 CH2 CH2 CH CH2 CH3 CH2 CH3 D. Petroleum 1. Petroleum – thick, dark liquid composed mostly of hydrocarbon compounds 2. Natural gas – consists mostly of methane, usually associated with petroleum deposits 3. To be used, petroleum must be separated by boiling into fractions. 4. Pyrolytic Cracking – heavier molecules of kerosene fraction are heated to 700oC, causing the fractions to break (crack) into gasoline fractions IV. Alkenes & Alkynes A. Naming Alkenes & Alkynes 1. Same rules for alkanes apply to alkenes & alkynes 2. Endings are different, and number the 1st carbon involved in the double/triple bond 3. Alkenes end in –ene; Example: 1-butene 4. Alkynes end in –yne; Example: 2-butyne B. Aromatic Hydrocarbon 1. Aromatic hydrocarbons – cyclic unsaturated hydrocarbons with strong aromas 2. Benzene – simplest aromatic hydrocarbon C. Isomers of Alkenes & Alkynes 1. Geometric Isomers: Isomers in which the order of atom bonding is the same but the arrangement of atoms in space is different Cis 1,2-dichloroethane Trans 1,2-dichloroethane 2. Enantiomers: mirror images of each other