Experiment 2: Density and Calibration
advertisement

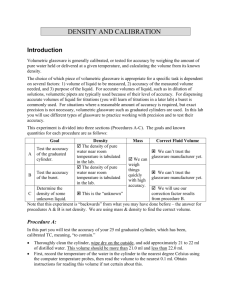
Experiment 2: Density and Calibration Introduction Volumetric glassware is generally calibrated, or tested for accuracy by weighing the amount of pure water held or delivered at a given temperature, and calculating the volume from its known density. The choice of which piece of volumetric glassware is appropriate for a specific task is dependent on several factors: 1. volume of liquid to be measured, 2. accuracy of the measured volume needed, and 3. 3) purpose of the liquid. For accurate volumes of liquid, such as in dilution of solutions, volumetric pipets are typically used because of their level of accuracy. For dispensing accurate volumes of liquid for titrations (you will learn of titrations in a later lab) a buret is commonly used. For situations where a reasonable amount of accuracy is required, but exact precision is not necessary, volumetric glassware such as graduated cylinders are used. In this lab you will use different types of glassware to practice working with precision and to test their accuracy. Each student will calibrate their own glassware. If you are not sure about a reading, first doublecheck your work, then ask your lab partner for an opinion. If you cannot resolve the question, consult your instructor. Procedure A Thoroughly clean the cylinder, wipe dry on the outside, and add approximately 21 to 22 ml of distilled water. This volume should be more than 21.0 ml and less than 22.0 ml. First, record the temperature of the water in the cylinder to the nearest 1 degree Celsius using the computer temperature probes, then read the volume to the nearest 0.1 ml, 1/10th of the smallest graduation. To correctly read the volume, refer to Appendix A of this manual for information regarding the meniscus. Weigh the cylinder and water on the balance to the nearest 0.001 gram. Remember that all containers must be clean and dry outside before they are weighed. Record your data in tabular form (table form) directly into your laboratory notebook as they are measured. Pour out the water, holding the cylinder inverted for 10 to 15 seconds to allow time for drainage. Do not wipe out the water or shake the cylinder. Reweigh the cylinder to the nearest 0.001 gram. Subtract the two masses to obtain the mass of the water. Repeat procedure A for a second trial, and then calculate the volume for each trial, using the density value from the density table posted on the laboratory bulletin board. The mass of the water divided by the density is equal to the volume calculated. Created by aherbelin 687294142 Page 1/6 2/6/2016 Compare the calculated volume with the volume read with the eye for each trial to obtain two corrections (calculated volume minus read volume). We will assume that the calculated volume is correct. (Is this a reasonable assumption?) If careful work has been done, the corrections should agree within 0.1 to 0.2 ml. At this point bring your notebook to the instructor for approval. If the agreement between the two trials is all right, then examine the relationship between the actual volume delivered and the volume read with the eye. This difference is known as a correction factor. Procedure B: Reading the Buret Add 5 mL of distilled water to rinse the buret. Carefully hold the buret horizontally while rolling it so the water rinses all of the interior surfaces. Pour this rinse water out through the top. Repeat. Fill the clean buret above the zero line with distilled water. Open the stopcock fully to dislodge all bubbles from the tip. Add more distilled water if necessary. NOTE: The buret needs to be full so the water pressure is high enough to dislodge bubbles. Determine the temperature of the water in the buret. Drain the water to bring the meniscus below the 0.00 mL line. Do not waste time getting exactly to the 0.00 ml line. Double check to be sure that the tip is filled with water and all bubbles are gone. Read the volume on the buret. You must estimate the reading to 0.01 mL, otherwise you will have to repeat this portion of the experiment. Drain between 40 and 41 ml of solution into a previously weighed (tared) bottle. The bottle should be weighed to the nearest 0.001 g. Allow 15-20 seconds for drainage time, and then read the volume to the nearest 0.01 mL. Use the paper card with a black line to help darken the meniscus as shown in Appendix C. A small hand magnifier will be helpful. Record your data in the notebook directly. Calculate the actual volume as in procedure B using the mass of the water delivered into the beaker. Compare the volume read with the volume calculated. This difference (calculated volume – read volume) is to be shown to the instructor. Run a second trial. Your correction factors must agree within +0.06 mL. Procedure C: Density of an Unknown Fluid. Obtain an unknown solution in a clean, dry beaker and determine its density. Develop your own procedure based on the buret method from procedure B. Be sure to account for your correction factor. Calculate the density using the correct number of significant figures. Repeat the experiment until your have two trials that agree within +0.004 g/ml. Take a clean dry 150 ml beaker to the stockroom to obtain an unknown solution. Created by aherbelin 687294142 Page 2/6 2/6/2016 Rinse the buret twice with 5 mL of the unknown solution. Fill the clean buret above the zero line with the unknown solution. Rinse the tip by dispensing 5-10 mL of the unknown solution. Record the initial volume of solution in the buret. Drain between 40 and 41 ml of the solution into a previously weighed bottle. (The bottle should be weighed on the analytical balance to the nearest 0.001 gram.) Allow about 15-20 seconds for drainage time, then read the volume to the nearest 0.01 ml. A small hand magnifier will be helpful. Weigh the bottle and the solution delivered by the buret to the nearest 0.001 gram. Record all data in the notebook directly. Use the buret reading that you find for the volume. Calculate the density of the unknown liquid by dividing the mass of solution by the volume. Report your answer in g/mL. Observe significant figures in all calculations. Run a second trial to show agreement. The difference between the densities calculated in the two trials cannot be greater than ±0.004. If it is, more trials must be run until two trials agree within the limit. Report: Complete the results section in your notebook. When you are done, obtain a copy of the Excel template from the instructor. Complete the report and submit the spreadsheet by email. Appendix A: Reading the meniscus When measuring the volume of a liquid, always read the scale from the bottom of the meniscus. The meniscus is the curved surface of a liquid in a narrow cylindrical container. When reading a scale, always strive to avoid parallax errors. Parallax errors arise when a meniscus or needle is viewed from an angle rather than from straight-on at eye level. The meniscus. Created by aherbelin 687294142 Correct: Viewing the meniscus Incorrect: viewing the meniscus at eye level. from an angle. Page 3/6 2/6/2016 Appendix B: Table of Densities of Water Density of Water at 1 atmosphere. (source: Handbook of Chemistry and Physics, CRC press, 64th Ed.) Temp. (ºC) Density (g/mL) Temp. (ºC) Density (g/mL) Temp. (ºC) Density (g/mL) 15 16 17 18 0.999103 0.998946 0.998778 0.998599 19 20 21 22 0.998408 0.998207 0.997996 0.997774 23 24 25 27 0.997542 0.997300 0.997048 0.996516 Appendix C: Using a Buret Card A buret card should have a black streak with a distinct horizontal zone of black against white. Electrical tape makes an excellent black zone. Name Created by aherbelin 687294142 When held behind the buret, the upper limit of the black streak should be placed just under the meniscus, so that the bottom of the meniscus can be seen distinctly against a narrow zone of white. ___________________ Note in this photo that the apparent level of the meniscus is different. Since placing the black streak just under the meniscus is more repeatable than at some variable distance, the close placement is recommended. Lab Section ___________________ Page 4/6 2/6/2016 Name ___________________ Lab Section ___________________ Pre-Lab Exercises Complete these exercises in your lab notebook then rewrite your work and the answers on this sheet to turn in. 1. Read fluid level in the following pictures (don’t put this one in your notebook): Volume: __________________ Volume ______________________ 2. A student calibrated a buret according to Procedure B with the following data: Mass of empty bottle: Mass of Bottle & H2O: Water Temperature: Buret Volume: Trial I 61.621 g 82.824 g 24 °C 21.26 mL Trial II 61.637 g 83.572 g 24 °C 21.99 mL If the density of water at 24 °C is 0.9973, what is the average correction value for the procedure? (You must show all of your work.) Created by aherbelin 687294142 Page 5/6 2/6/2016 Average Correction Value: __________ 3. The student used the same buret to find the density of an unknown solution with the following data: Trial I Trial II Mass of empty bottle: 57.265 g 57.267 g Mass of bottle & unknown: 88.217 g 85.332 g Volume on buret: 42.67 mL 38.68 mL What is the average density of the unknown? Be sure to use the buret’s correction value from problem #1. (You must show all of your work.) Average Density: __________ Lab Preparation Checklist: Title, Purpose, and Procedure hand-written in notebook. Table of Contents in notebook. Prelab calculations completed, showing work. Data tables prepared. Bring: proper clothes, goggles, bound laboratory notebook, pen, pencil (for glassware) Created by aherbelin 687294142 Page 6/6 2/6/2016