DENSITY AND CALIBRATION
advertisement

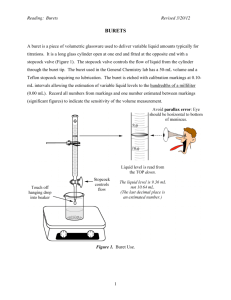
DENSITY AND CALIBRATION Introduction Volumetric glassware is generally calibrated, or tested for accuracy by weighing the amount of pure water held or delivered at a given temperature, and calculating the volume from its known density. The choice of which piece of volumetric glassware is appropriate for a specific task is dependent on several factors: 1) volume of liquid to be measured, 2) accuracy of the measured volume needed, and 3) purpose of the liquid. For accurate volumes of liquid, such as in dilution of solutions, volumetric pipets are typically used because of their level of accuracy. For dispensing accurate volumes of liquid for titrations (you will learn of titrations in a later lab) a buret is commonly used. For situations where a reasonable amount of accuracy is required, but exact precision is not necessary, volumetric glassware such as graduated cylinders are used. In this lab you will use different types of glassware to practice working with precision and to test their accuracy. This experiment is divided into three sections (Procedures A-C). The goals and known quantities for each procedure are as follows: Goal A Test the accuracy of the graduated cylinder. Density The density of pure water near room temperature is tabulated in the lab. The density of pure water near room temperature is tabulated in the lab. Mass Correct Fluid Volume We can’t trust the glassware manufacturer yet. We can weigh things We can’t trust the Test the accuracy quickly B glassware manufacturer yet. of the buret. with high accuracy. We will use our Determine the correction factor results C density of some This is the “unknown” from procedure B. unknown liquid. Note that this experiment is “backwards” from what you may have done before – the answer for procedures A & B is not density. We are using mass & density to find the correct volume. Procedure A: In this part you will test the accuracy of your 25 ml graduated cylinder, which has been, calibrated TC, meaning, “to contain.” • • Thoroughly clean the cylinder, wipe dry on the outside, and add approximately 21 to 22 ml of distilled water. This volume should be more than 21.0 ml and less than 22.0 ml. First, record the temperature of the water in the cylinder to the nearest degree Celsius using the computer temperature probes, then read the volume to the nearest 0.1 ml. Obtain instructions for reading this volume if not certain about this. • • • • Weigh the cylinder and water on the balance. Remember that all containers must be clean and dry outside before they are weighed. Record your data in tabular form (table form) directly into your laboratory notebook as they are measured. Pour out the water, holding the cylinder inverted for 10 to 15 seconds to allow time for drainage. Do not wipe out the water or shake the cylinder. Reweigh the cylinder. Subtract the two masses to obtain the mass of the water. Repeat procedure A for a second trial, and then calculate the volume for each trial, using the density value from the density table posted on the laboratory bulletin board. The mass of the water divided by the density is equal to the volume calculated (Vc). Compare the calculated volume with the volume read with the eye (Vr) for each trial to obtain two correction factors (CF = Vc – Vr). We will assume that the calculated volume is correct. (Is this a reasonable assumption? Why or why not?) If careful work has been done, the corrections should agree to 0.1 to 0.2 ml. At this point bring your notebook to the instructor for approval. If the agreement between the two trials is all right, then examine the relationship between the actual volume delivered and the volume read with the eye. This difference is known as a correction factor. Procedure B: In this part you will test your ability to read a buret. Instructions will be given on the care and use of a buret. • • • • • • • • • Fill the clean buret above the zero line with distilled water. Determine the temperature of the water in the buret. Drain out enough of the water so that the meniscus comes exactly on the 0.00 ml line. Be sure that the tip is filled with water and that all bubbles are gone. Drain between 40 and 41 ml of solution into a previously weighed bottle. (The bottle should be weighed on the balance to the nearest 0.001 gram.) Allow about 15-20 seconds for drainage time, then read the volume to the nearest 0.01 ml. A small hand magnifier will be helpful. Weigh the bottle and the solution delivered by the buret to the nearest 0.001 gram. Record all data in the notebook directly. Calculate the actual volume as before using the density data. Compute the correction factor (CF) and show it to the instructor. Run a second trial. Your correction factors (CF) must agree within +0.06 mL. Repeat Procedure B until two correction factors agree. Procedure C: • • • Take a clean dry 150 ml beaker to the stockroom to obtain an unknown solution. Fill the clean buret above the zero line with the unknown solution. (You do not need to determine the solution's temperature.) Drain out enough of the solution so that the meniscus comes exactly on the 0.00 ml line. Be sure that the tip is filled with the solution and that all bubbles are gone. Drain between 40 and 41 ml of the solution into a previously weighed bottle. (The bottle should be weighed on the analytical balance to the nearest 0.001 gram.) • • • • • • Allow about 15-20 seconds for drainage time, then read the volume to the nearest 0.01 ml. A small hand magnifier will be helpful. Weigh the bottle and the solution delivered by the buret to the nearest 0.001 gram. Record all data in the notebook directly. Use the buret reading that you find for the volume. Calculate the density of the unknown liquid by dividing the mass of solution by the volume. Report your answer in g/mL. Observe significant figures in all calculations. Run a second trial to show agreement. The difference between the densities calculated in the two trials cannot be greater than ±0.004. If it is, more trials must be run until two trials agree within the limit. Chemical Waste: The unkown solution is simply a salt (NaCl) solution and can be poured down the sink drain. Report: Using the data recorded in your notebook, write up a report for procedures B and C in ink on engineering report paper. Report the results of your individual trials and for procedure C also calculate and report the average density. Follow the format given in the sample report given earlier. Pre-Lab Exercise: Name: ____________ Complete the following calculations in your lab notebook. Clearly identify each answer. After you finish, rewrite prelab calculations (showing work) on this sheet to turn in before lab starts. 1. A student calibrated a buret according to Procedure B with the following data: Trial I Trial II Mass of empty bottle: 61.621 g 61.637 g Mass of Bottle & H2O: 82.824 g 83.572 g Water Temperature: 24 °C 24 °C Buret Volume: 21.28 mL 22.04 mL If the density of water at 24 °C is 0.9973, what is the average correction value for the procedure? (You must show all of your work.) Average correction value: 2. The student used the same buret to find the density of an unknown solution with the following data: Trial I Trial II Mass of empty bottle: 57.265 g 57.267 g Mass of bottle & unknown: 88.217 g 85.332 g Volume on buret: 42.67 mL 38.68 mL What is the average density of the unknown? Be sure to use the buret’s correction value from problem #1. (You must show all of your work.) Average density: Lab Preparation Checklist: Table of Contents on pages 1 in notebook. All pages in lab notebook are numbered (at least the first 50!). Title, Purpose, and Procedure hand-written in notebook. Prelab calculations completed, showing work. Data tables prepared. Bring: proper clothes, goggles, bound laboratory notebook, pen, pencil (for glassware)