PROP 383 - Le Moyne College
advertisement

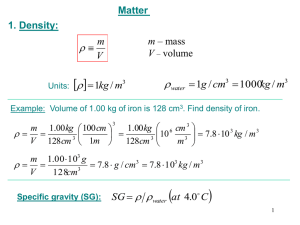
Lab 3 Determination of Density of Liquids and Solids Uncertainties in Scientific Measurement Systematic Errors (Determinate Errors) • Bias an experimentally determined value in one direction • Have a definite value and an assignable cause • Influence experimental accuracy • May be avoided altogether OR measured and corrected (“correction factor”) Uncertainties in Scientific Measurement Random Errors (Indeterminate Errors) • Errors may be high or low • Arise from uncertainties in measurement that are either unknown or uncontrollable by the experimenter • Effect experimental precision Reducing Your Error (Mass measurements) • Do not touch the object to be weighed with bare hands • Clean the outside of object to be weighed (wipe; wash & dry), prior to weighing • Tare the balance to avoid recording an irrelevant mass (e.g., the container) Reducing Your Error (Volume Measurements) • Carefully read volumes: Avoid parallax error! • Carefully transfer fluids (or an object into fluid) to avoid splashing; tilt container containing the fluid for easier transfer • Gently tap side of fluid container to dislodge air bubbles Parallax Error occurs when you read the volume anywhere BUT at the “bottom” of the meniscus (for most liquids) or “top” of the meniscus (for mercury). CORRECT (“true”) reading is at the meniscus (“curve”) in the center of the cylinder. Actual Densities • Rubbing alcohol and Rubber stopper: correct densities will not be given • Metal: correct densities of all unknowns will be posted Remember significant figures!!!! Do problem and adjust (round) final answer.