Physical Properties/Density determination Lab
advertisement

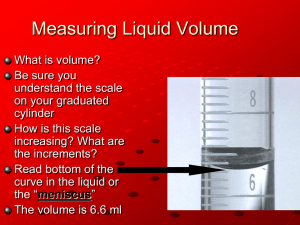
page1 of 11 Version 2003 Density Determination. Measuring A Physical Property. Objective: Measurement of the density of water. . Assessing the precision and accuracy of some common laboratory measurements. Introduction: As soon as you were able to distinguish yourself from the rest of the world you were studying the physical properties of compounds. Physical properties include color, hardness, melting point, boiling point, density, odor, refractive index, solubility and electrical conductivity, to name a few. In this laboratory experiment you will measure physical properties of several compounds. You will use these measurements to assess the accuracy of your thermometer, and the precision of your weighing and volumetric technique. The physical properties of several thousand compounds are tabulated in: The Handbook of Chemistry & Physics, nth ed. (R.C.Weast, Ed.), CRC Press, Boca Raton, FL. The Merck, Index, ith ed. (Martha Windholz, Ed.), Merck and Co., Rahway, NJ. Please note that these are not complete citations. There are several editions of these references in circulation. You are responsible for citing your source correctly. MEASUREMENT AND STATISTICS: All experimental science involves taking measurements. Human eyes, ears, tastebuds, and other sensory neurons, the first "instruments", can be trained to really quite remarkable levels of sophistication in measurement. For example, Martina Navratilova can detect a difference in the mass of her tennis racket of only 50 mg., the mass of a postage stamp. In general, however, one's senses are supplemented by manufactured, calibrated instruments. One's measurements are only as good as the instrument allows them to be. Every instrument has inherent limitations, and thus, some error. Good, limitation, and error are value-laden terms. Let us understand exactly what we mean by them in the context of the laboratory. A measurement is a quantitative description of a fundamental physical property, compared to a standard. To a scientist, a good measurement is one which is both accurate and precise. An accurate measurement is one in which the measurement you get from reading the instrument is very close to the "true" value. The true value of a measurement is what the quantity being measured actually is.1 The error of a measurement is usually expressed as the percent difference between the measured value and the true value. A measurement is said to be precise if a each one of a series of measurements agrees closely with the others. In the world on the other side of the quad, precision and accuracy are used as synonyms. In science, precision may or may not be related to accuracy. Precision is determined statistically, usually as the mean or average of all measurements, +/- the standard deviation of the data from that mean. Example: You win the National Bureau of Standards sweepstake, and receive a bar of platinum. This was the original "Centimeter of the Archives", and is set to be exactly 1.0000000...(an infinite # of 0's) centimeter long. This is the true value. You and your colleagues set out to measure the bar with a yardstick. Your stick says that the bar is 1.010 cm long. This represents an absolute error in measurement of: Absolute error = | true value - measured value | 1 Of course, the absolute "true value" is not known to an infinite number of decimal places except by God. page2 of 11 Version 2003 = | 1.00000000...cm - 1.010 cm | = 0.010 cm. You may express this as a percent: % absolute error = | true value - measured value | . 100 true value = | 1.0000...cm - 1.010 cm | . 100 = 1.00% 1.0000… cm Data Set 1 1.010 cm 0.998 cm 1.002 cm 1.011 cm 0.996 cm You and your colleagues do several measurements, with the following results: The average is: [ Σxi]/n = 1.010cm + 0.998 cm + 1.002cm + 1.011cm + 0.996 cm 5 = 1.003 cm = ā (“¯” “bar” is the symbol for "average" or "mean" The precision, roughly how far each experimental value is from the average, is given by the standard deviation. s.d. = √∑ | xi - ā | 2 /n-1 = 0.006119 s.d..100/ ā = variation in the data = [0.006119.100] /1.003 = 0.610 % (aka the "% error") The standard deviation and the variation are measures of precision. The smaller the s.d. and variation, , the better the data “agree”. You now use two other rulers and obtain two more sets of data: ****************************************************************** Data Set 2 2.0000 cm 1.9980 cm 2.0010 cm 2.0010 cm 1.9990 cm ā = 1.9998 cm % absolute error = [mean - true value]∙100/true value = = [1.9998 cm - 1.0000... cm] . 100 /1.000...cm = 199.98% error Not very accurate! Standard deviation = 1.166 x 10-3 cm % error = [s.d. ∙ 100]/ ā = [1.166 x 10-3 cm . 100] / 1.9998 cm = 0.058% Very precise! Data Set 3 1.3 cm 0.7 cm 1.2 cm 0.8 cm 1.0 cm ****************************************************** ā = 1.0 cm % absolute error = [mean - true value]∙100/true value = = [1.0 cm - 1.0000... cm] . 100/1.0000...cm = 0% error. Very accurate! Standard deviation = 0.23 cm % error = [s.d/ ā.] ∙ 100 = [0.23 cm / 1.0 cm] . 100 = 23% Not very precise! ************************************************************ Data from set 2 (some practical joker made a ruler in which an inch was 1.27 cm, instead of 2.54 cm) is very precise, but rather inaccurate. Data from set 3 (your friends were using their kindergarten rulers) is very accurate (by luck!) but not very precise. page3 of 11 Version 2003 BEFORE YOU COME TO LAB: IN YOUR OWN WORDS, EXPLAIN THE DIFFERENCE BETWEEN ACCURACY AND PRECISION. SHOW HOW TO CALCULATE EACH ONE. PHYSICAL PROPERTIES: Mass: (From the Greek - to knead) - As Plato knew, that which everyone knows is often rather difficult to define. Mass is defined as the amount of matter in a sample of any substance. Matter is the tangible, touchable, sensible stuff of the universe…anything which has mass and takes up space. All of which is very well, but how does one measure the mass of matter? How do you know that an apple has more mass than, say, a Hersey bar? Well, the apple weighs more than the chocolate. At least, it does over at Alliot Hall. But what if you are up in the space shuttle, where both are weightless? Does everything have zero mass in space? That seems to fly in the face of logic. A better definition of mass is that it is a measure of inertia – inertia being the tendency of a body at rest to remain at rest until it gets pushed. When a push, or force, does move an object, it accelerates. More mass = more inertia. It takes a force to overcome inertia. This leads us to a functional way to measure mass. Typically, we measure the mass of an object taking into account that it is accelerating…In Cheray 219, that acceleration is the acceleration due to the earth’s gravity. The weight of an object is equal to its mass times the acceleration due to gravity, w = mg, where m is mass, and g is the acceleration due to gravity at the earth’s surface, 9.8 m/sec2. Volume: Since matter lives in space, it has a volume. A volume is simple that amount of space a given substance occupies. Volume has units of length cubed. For example, a 1.00 milliliter (mL) volume is a cube 1.00 centimeter (cm) high, deep and wide; 1.00 cm3. A coffee cup holds about 200 mL (200 cm3) volume. Density: The density of substance is defined as the mass of a given volume of that substance. The units of density are g/mL or mg/μL. (Note: g/mL, g/cm3, and mg/μL are THE SAME THING) What a density of 0.9765g/ml tells you is that 0.9765 grams of that substance has a volume of 1 mL. Saying the same thing, 0.9766 mg of that substance has a volume of 1 μL. Write it this way: 0.9765 g stuff 1 mL stuff You can also write it: 1 ml stuff 0.9765 g stuff Experimentally, if you know the mass of a solid, liquid or gas (you weigh it), and the amount of space it takes up (its volume), you can combine these data and find the density. Density can give you some useful information. For example: Solid lead has a density of 11.34 g/cm3 at room temperature. Mercury, the only metal liquid at room temperature, has a density of 13.534 g/mL at 25oC. Pure room temperature water has a density of 0.997 g/mL at 25oC. Lead sinks like a stone in water, but floats in mercury. Melting Point: page4 of 11 Version 2003 The melting point of a pure element or compound is: that temperature at which the solid and liquid phase of the element or compound are in equilibrium. The melting points of different elements and compounds is related to the strength of interatomic, intermolecular, or interionic forces holding the solid together. Experimentally, if you slowly heat a solid element or compound (1◦C/minute is what you should aim for), the temperature of the system will rise until the first drop of liquid is formed. The temperature of the system will rise until the first drop of liquid is formed. The temperature will stay constant until all the solid is melted and in the liquid phase. Then the temperature will continue to rise. Boiling Point: The boiling point of a pure liquid element or compound is defined as that temperature at which the liquid and vapor phase of the compound are in equilibrium. This boiling temperature is dependent on the pressure of the system. Boiling points are typically obtained at 1.00 atmosphere pressure (760 mm Hg) unless otherwise indicated. The boiling points of different elements and compounds are related to the strength of interatomic, intermolecular, or interionic forces holding the liquid together. Experimentally, what this means is that, if you heat a liquid in a container open to the atmosphere, e.g., a bucket, a beaker, a test tube, or a reflux condenser, the temperature will continue to rise until bubbles of vapor form. This is boiling, and the temperature is the boiling point. The temperature will then remain constant until all the liquid has boiled away. !) but not very precise. BEFORE YOU COME TO LAB: IN YOUR OWN WORDS, DEFINE MASS, VOLUME, DENSITY, MELTING POINT AND BOILING POINT. EXPLAIN HOW TO MEASURE EACH. page5 of 11 ON TO THE GOOD PART: Version 2003 EXPERIMENT: The point of this experiment is to get a handle on the accuracy (and limitations thereof!) of some commonly used measuring instruments. You will use an analytical BALANCE and a Top-Loading Preparatory BALANCE to measure mass. By the way: Scientific instruments designed to measure mass are called BALANCES . A BALANCE is designed to balance the mass of an unknown object directly against a known mass… A scale is a calibrated spring. You have scales in the bathroom, but NOT in the laboratory! THIS ↓ IS A TOP-LOADING BALANCE! IT IS ACCURATE TO +/- 0.01 g. The +/0.01 grams is the instrument’s PRECISION. A very rough way to think about precision: This balance can measure the difference in mass between an object with mass 8.92g and another object with mass 8.93g. It CANNOT measure a difference in mass between an object with mass 8.933 g and an object with mass 8.934 g. ↑ THIS IS AN ANALYTICAL BALANCE! IT IS ACCURATE TO 0.1 mg (+/-0.0001 g), the PRECISION) Another way to think about precision: If you place an object on this balance and get a reading of 1.9714 g, you can be confident that next time you take the mass of that same object, you will get readings of 1.9713g, 1.9714 g, or 1.9715 g. THIS ↑ IS A BATHROOM SCALE Note that Precision is NOT accuracy. Precision is a measure of how closely a whole bunch of readings of the same thing will agree with each other. ACCURACY is a measure of how closely the readings come to the “real thing”. Or balances are calibrated annually, against “known” masses, by a technician from the factory. The technician has a set of weights whose masses are agreed upon page6 of 11 You will also use several glassware instruments designed to measure volume: Version 2003 1. A beaker: . This is a crude way to measure volumes! !) but not very precise. ESTIMATE HOW PRECISE YOUR BEAKER IS. 2. A graduated cylinder: A better way to measure volumes are TO DELIVER (TD) graduated cylinders…”To Deliver” glassware is designed to dispense a known volume of liquid into another container. This is as opposed to “To Contain” (TC) glassware, which is designed to Hold a known volume of liquid. (See volumetric flasks). THE PRECISION OF A 10 mL GRAD. CYLINDER is +/-0.1 mL. TECHNIQUE! When using volumetric glassware, the meniscus is what you want to look at. The meniscus is the curved surface of the liquid. meniscus For consistency, always: 7. Put the bottom of the meniscus on the volume line, when using a graduated cylinder to measure a desired volume; 8. Read the volume line at the bottom of the meniscus; 9. Avoid parallax error Look at the meniscus straight on! (What is “parallax error?” Things appear different depending on where you look at them. Try this: Look at an object about 3 meters away from you with just your right eye. Now close your right eye and look at the object just with the left eye. See how the object jumps around? Try this when reading a pipet or buret. Look at the meniscus from above, below, and at eye level…you’ll get 3 different readings! For consistency, always take readings with the meniscus at eye level…you may have to do some bending, stretching or standing on benches to do this, but DO IT! page7 of 11 Version 2003 3. A Buret: Burets are also “To Deliver” glassware, designed to accurately dispense varying amounts of liquid. The precision of our burets is +/- 0.05 mL 4. 5. 6. 7. 8. 9. 10. TECHNIQUE: When using volumetric glassware, the meniscus is what you want to look at. The meniscus is the curved surface of the liquid. meniscus For consistency, always: 4. Put the bottom of the meniscus on the volume line, when using a volumetric flask or pipet; 5. Read the volume line at the bottom of the meniscus, when using a buret; 6. Avoid parallax error Look at the meniscus straight on! For consistency, always take readings with the meniscus at eye level…you may have to do some bending, stretching or standing on benches to do this, but DO IT! page8 of 11 4. Version 2003 A volumetric flask: A “To Contain” (TC) piece of glassware. This glassware is designed to hold a fixed volume of liquid. When you pour the liquid out, all bets are off! THE PRECISION OF OUR VOLUMETRIC FLASKS IS: 10 mL flasks: +/- 0.02 mL 25 mL flasks: +/- 0.03 mL 100 mL flasks: +/- 0.08 mL Volume scratch on the neck TECHNIQUE! When using volumetric glassware, the meniscus is what you want to look at. The meniscus is the curved surface of the liquid. For consistency, always: 1. Put the bottom of the meniscus on the volume line, when using a volumetric flask or pipet; 2. Read the volume line at the bottom of the meniscus, when using a graduated cylinder or buret; 3. Avoid parallax error Look at the meniscus straight on! (What is “parallax error?” Things appear different depending on where you look at them. For consistency, always take readings with the meniscus at eye level…you may have to do some bending, stretching or standing on benches to do this, but DO IT! page9 of 11 5. A volumetric pipet. A “To Deliver” (TD) piece of glassware. The precision of our pipets is approximately +/- 0.01 mL. Version 2003 Volume scratch on the neck. TECHNIQUE: An exercise in hand-finger-eye coordination! NEVER pipet by mouth! ALWAYS use a blue bulb or pump; ALWAYS keep the pipet straight up & down. Don’t tilt it! Use your most coordinated hand (Left for left-handers, right for right-handers) to hold the pipet. Use your other hand for the bulb. Always bring the liquid level up past the scratch on the pipet neck, and then.. 1. put your coordinated index finger (not thumb) over the neck to control the flow; 2. by releasing pressure on your index finger, let the liquid drain until; 3. the meniscus bottom is sitting on the scratch. 4. Put more pressure on your index finger to stop the flow. Now, when you are ready to dispense the liquid, release your index finger, and, keeping the pipet straight up & down, let the liquid flow into the new container. When it has all drained; Don’t blow out the rest of the liquid, just tough the tip to the side of the container. page10 of 11 2003 Version Your task: Determine the density of pure water at 0oC using a variety of methods. Determine which method is most precise. Determine which method is most accurate. Method 1 2 3 4 5 6 7 8 9 10 11 12 Mass mesurement Top-loader Top-loader Top-loader Top-loader Top-loader Top-loader Analytical balance Analytical balance Analytical balance Analytical balance Analytical balance Analytical balance Volume measurement 10 mL beaker 10 mL grad cylinder Buret 25.00 mL volumetric flask 1.00 mL volumetric pipet 5.00 mL volumetric pipet 50 mL beaker 10 mL grad cylinder Buret 25.00 mL volumetric flask 1.00 mL volumetric pipet 5.00 mL volumetric pipet Put a large beaker of distilled water in an ice bath to cool. Record the temperature with a thermometer. Use the ice water for all determinations. Your instructor will tell you which of the above 12 methods to follow. Check out all your instruments. Record (or estimate) the precision of each one. Determine the mass and volume of water using each method at least 3 times. Record this data as you obtain it, with the correct number of significant figures. Be sure you use the same balance for each measurement! Calculate the average density of 0oC water for each method. Calculate the standard deviation and % error (sd x 100/average, the precision) for each method. Look up the density of 0oC water in the Handbook of Chemistry and Physics. Record it, and cite the source! No reference, or incorrect reference, results in a HUGE score reduction. Calculate the absolute error for each method ([mean - true value]∙100/true value, the accuracy). Analyze your data to determine which method is the most accurate. See below for a sample data and results sheet. page11 of 11 2003 Version Always include a SUMMARY TABLE OF RESULTS: NOTICE THAT THE “HANDBOOK OF CHEMISTRY AND PHYSICS, 100th edition” is not due out for 15 years. WRITE DOWN THE EDITION YOU USED.