Class Activity 06_Solution
advertisement
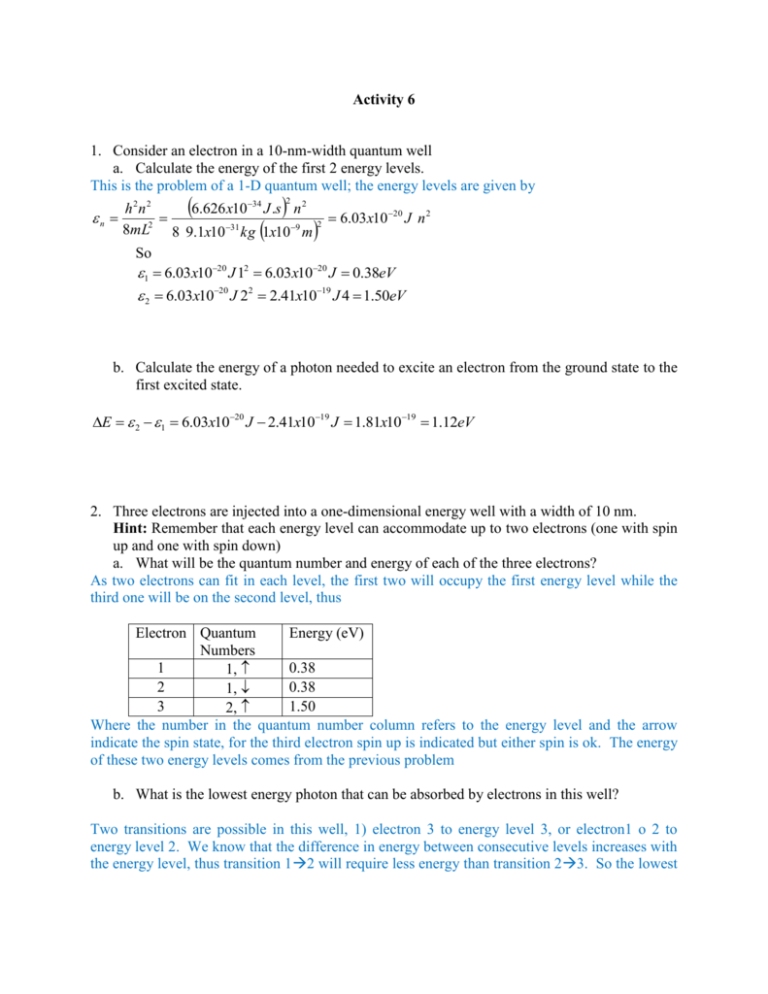
Activity 6 1. Consider an electron in a 10-nm-width quantum well a. Calculate the energy of the first 2 energy levels. This is the problem of a 1-D quantum well; the energy levels are given by 2 h2n2 6.626 x10 34 J .s n 2 n 6.03x10 20 J n 2 8mL2 8 9.1x10 31 kg 1x10 9 m 2 So 1 6.03x1020 J 12 6.03x1020 J 0.38eV 2 6.03x1020 J 22 2.41x1019 J 4 1.50eV b. Calculate the energy of a photon needed to excite an electron from the ground state to the first excited state. E 2 1 6.03x1020 J 2.41x1019 J 1.81x1019 1.12eV 2. Three electrons are injected into a one-dimensional energy well with a width of 10 nm. Hint: Remember that each energy level can accommodate up to two electrons (one with spin up and one with spin down) a. What will be the quantum number and energy of each of the three electrons? As two electrons can fit in each level, the first two will occupy the first energy level while the third one will be on the second level, thus Electron Quantum Energy (eV) Numbers 1 0.38 1, 2 0.38 1, 3 1.50 2, Where the number in the quantum number column refers to the energy level and the arrow indicate the spin state, for the third electron spin up is indicated but either spin is ok. The energy of these two energy levels comes from the previous problem b. What is the lowest energy photon that can be absorbed by electrons in this well? Two transitions are possible in this well, 1) electron 3 to energy level 3, or electron1 o 2 to energy level 2. We know that the difference in energy between consecutive levels increases with the energy level, thus transition 12 will require less energy than transition 23. So the lowest energy a photon must have to produce a transition is equal to the difference in energy between levels 1 and 2 that was calculate in the previous problem to be, 1.12eV. Of course transition between non-consecutive levels is possible but will most probably require more energy than a transition between consecutive levels c. Would the energy of the lowest energy photon that can be absorbed be different if there were 2 instead of 3 electrons? What if there were 4? If there were only two electrons, they will occupy the lowest energy level, then the only possible transition between consecutive levels will be 12, thus the smaller photon energy is the same as above. If there are 4 electrons then both levels, 1 and 2 and full so the only possible transition between consecutive energy levels is between levels 2 and 3, thus we will need to know the energy of level 3, 3 6.03x1020 J 32 5.43x1019 J 3.39eV Thus the smallest photon energy must be E 3 2 5.43x1019 2.41x1019 J 3.02 x1019 1.89eV