Electronic Structure of Atoms
advertisement

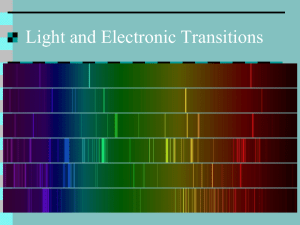
Electronic Structure of Atoms Name______________________________ Date___________ Block____ Wave Nature of Light We are familiar with water waves: drop a stone into a quiet pool of water and the impact of the stone with the water starts an up-and-down motion of the water surface. A wave is a continuously repeating change in matter or in a physical field. (A physical field is a region in which a form of energy such as magnetism is present.) All waves to which we will refer are transverse waves. A transverse wave travels in one direction but moves back-and-forth across (transverse) the line of travel. A transverse wave has two “parts”: the crest (“top” of the wave) and the trough (“bottom” of the wave). See diagram below. Wavelength (λ) Trough Waves generally are characterized by two properties: wavelength and frequency. Wavelength is the distance between a point on one wave and the same point on the next or adjacent wave. Frequency is the number of waves that pass a given point during one unit of time (usually 1 second). Wavelength and frequency are inversely related: as wavelength decreases, the frequency increases. Frequency is directly related to the energy of the wave: as frequency increases, the energy of the wave increases. Another property of waves is amplitude. Amplitude is sometimes called the “wave height” and is the distance from either the crest or trough to an imaginary line drawn midway between the crest and trough. Amplitude is not related to either wavelength or frequency. See diagram above for a labeled transverse wave. 1 Many forms of energy such as X-rays, gamma rays, ultraviolet light, visible light, infrared light, heat, radio waves, microwaves, and television waves, behave as waves and have specific wavelengths and frequencies. Because these forms of energy involve changes in electric and magnetic fields, they are called electromagnetic radiation. (Remember: radiation is simply energy that travels outward in all directions (i.e., radiates) from the energy source.) Note: electrical energy and magnetism are never found separately: a magnetic field exists anywhere there is electrical energy; an electrical field exists anywhere there is magnetism. Regions of the Electromagnetic Spectrum The electromagnetic spectrum is the range of frequencies or wavelengths of electromagnetic radiation. In order by increasing frequency (and decreasing wavelength): TV/FM radio waves; radar; microwaves; heat / infrared; visible light; ultraviolet; X-rays; gamma rays; cosmic rays. See Figure 10-5 in your textbook for a diagram of the electromagnetic spectrum. Bright-line Spectrum The visible light spectrum is only a very small region of the entire electromagnetic spectrum. Visible light extends from the violet end having a wavelength of about 400 nanometers (4 x 10 –9 m) to the red end having a wavelength of less than 800 nm (8 x 10 –9 m). Any light waves having wavelengths shorter than 400 nm or longer than 800 nm are not visible to the human eye. Infrared radiation (infra- means “below”) has wavelengths greater than 800 nm. Ultraviolet radiation (ultra- means “beyond”) has wavelengths less than 400 nm. Each color of light has its own specific wavelength and frequency. When visible light from some source is passed through a prism, the light is separated into distinct bands of color. If “white” light (containing the entire visible spectrum) is passed through a prism, the light is separated into a continuous spectrum (no “breaks” between the colors) or what we call a “rainbow.” Very often, a source of visible light does not emit the entire visible spectrum. Some wavelengths of light are not present. Sometimes, a visible light source emits only a few wavelengths of light. The few wavelengths of visible light are observed as bright lines of color. The specific bright lines are the bright-line spectrum for that source of visible light. See Figure 10-7 on page 338 in your textbook for example of visible light that has not been passed through a prism (unrefracted light), a continuous spectrum, and three examples of bright-line spectra. 2 An element can be heated to a high enough temperature that specific wavelengths of visible light are emitted. These wavelengths are the bright-line spectrum for that particular element and the element can be identified by its bright-line spectrum. Quantized Energy The energy of visible light is said to be quantized: i.e., the possible energies are limited to certain values and to no other values. All light of the same wavelength has one specific energy value. Remember from the Modern Model of the Atom that particles could have properties of waves. Remember also that Niels Bohr proposed that electrons could move from one level to a higher level if the electron absorbed a specific amount of energy. Bohr also stated that energy would be emitted by the electron when the electron returned to its original level. There is experimental evidence that demonstrates that electrons (subatomic particles) can and do behave as waves. There is evidence that demonstrates that electrons can and do move from a lower level to a higher level if they absorb specific amounts of energy. If the specific amount of energy is NOT absorbed, the electron does not move to the higher energy level. One way electrons in atom can absorb energy is through the collision of two atoms. During the collision, kinetic energy from one atom can be gained by one or more electrons of the other atom thereby boosting or exciting the electron from its normal lower energy level to a higher energy level. When the electron is in the higher energy level, the electron is said to be “excited.” Another way of exciting electrons is to add energy to the atoms by heating a sample of matter. Also, some chemical reactions may release enough energy to excite electrons. As the excited electron returns to its original energy level (called a transition), the electron loses energy which is emitted as a “packet” of energy called a photon. The energy of the photon can be calculated by subtracting the lower energy of the electron from the higher energy of the electron. The photon can behave as either a wave or as a particle: how the photon behaves depends on how we – the observer – interact with it (we interact with things by observing them such as when we take measurements). We can see this photon when it behaves as a wave and has a wavelength within the visible light spectrum. When the visible light energy is passed through a prism, a bright-line spectrum can be seen. Since each element has its own bright-line spectrum, even a compound containing several elements can be analyzed as to which elements make up the compound. Energy Levels and Sublevels of Electrons in an Atom A principal energy level in the electron cloud is a general region in which electrons having certain energies may be found. The principal energy levels are numbered: 1,2,3,4,5,6,7, etc. In 3 atoms of the known elements, 7 principal energy levels have been identified as containing electrons. Within each principal energy level are one or more sublevels. Each sublevel contains electrons having certain energies. The sublevels that have been identified are s, p, d, and f. Within each sublevel are one or more orbitals. Each orbital contains a maximum of two (2) electrons. The orbitals are designated by their relative location in three dimensions depending along which axis the orbitals lay (x, y, or z). See the table that follows for the first four principal energy levels, available sublevels in each principal energy level, number of orbitals in each sublevel, and maximum number of electrons per sublevel. Table of the First 4 Principal Energy Levels: Sublevels, Number of Orbitals, and Maximum Number of Electrons in Each Sublevel Principal Energy Level Available Sublevels 1 s 1 2 2 s p 1 3 2 6 s p d 1 3 5 2 6 10 s p d f 1 3 5 7 2 6 10 14 3 4 Number of Orbitals Maximum Number of Electrons per Sublevel There are currently 7 principal energy levels that have been identified. However, only four (4) sublevels have been identified as containing electrons: s, p, d, and f. An electron enters the lowest energy principal energy level available. Within that principal energy level, the electron will enter the lowest energy sublevel available. Within that sublevel, the electron will enter the lowest energy orbital available. Once every available orbital in a given sublevel contains one (1) electron, a second electron may then enter an orbital to form a pair of electrons in the orbital. The following Aufbau diagram illustrates the order of filling energy levels, and sublevels. Start at 1s. Follow each arrow from the back of the arrow to the tip. The order of filling sublevels is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 6f, 7d, 7f. 4 7s 7p 7d 7f 6s 6p 6d 6f 5s 5p 5d 5f 4s 4p 4d 4f 3s 3p 3d 2s 2p 1s 5