Emission Spectra and Flame Tests
advertisement

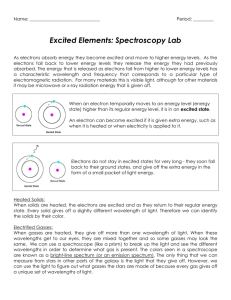
Light and Electronic Transitions The Big Questions What is light? How is light emitted? What do electrons have to do with light? What are emission spectra? How do flame tests help identify metals? The Electromagnetic Spectrum All light is part of the EM spectrum. Most is invisible: gamma, X-rays, UV, IR, microwaves, radio waves Visible light: wavelength (w.l.) from 400 to 700 nm. The EM Spectrum EM Radiation Light is a carrier of energy. Energy is proportional to frequency. Frequency is inversely proportional to wavelength. Longer wavelength = lower frequency = lower energy. Shorter wavelength = higher frequency = greater energy. Electrons and Quanta Ground state – the lowest energy position an e- can occupy. Excited state – a temporary high-energy position. Quantum (pl. quanta) – the amount of energy needed to move an e- to a higher energy level. Electrons and Quanta If an atom absorbs exactly 1 quantum of energy, an electron can be boosted from a ground state to an excited state. The electron is only in the excited state for a very short period of time. Soon the e- returns to its ground state and emits the quantum of energy as light. In some cases the emitted light is in the visible spectrum. Light and Electrons Excited state (E.S.) electron Ground state (G.S.) electron Light and Electrons Excited state (E.S.) electron 1 quantum Ground state (G.S.) electron Light and Electrons Emission Spectrum Emission spectrum – wavelengths of light given off by an element when it is excited (usu. by heat). Every element has unique emission spectrum. Emission Spectra Hydrogen Helium Carbon Flame Tests Flame test – used to ID some metals in compounds. Each metal gives a flame a characteristic color. Can identify metals based on flame colors.