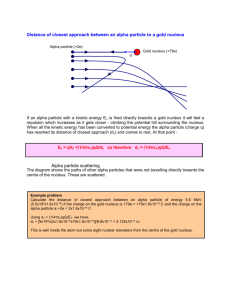
particle atomic
advertisement

NUCLEUS & RADIOACTIVITY QUESTIONS and ANSWERS 1. Why is the mass of an atom practically equal to the mass of its nucleus? Protons and neutrons are about 2000 times more massive than the electrons. 2. What are quarks? The six quarks (up, down, charm, strange, top and bottom) are fundamental particles which (along with the leptons) make up (almost) all particles in nature. Quarks are especially important in that three quarks form protons and neutrons. 3. Name three different leptons. Electron, muon, tau, and their neutrinos. 4. Why do protons in a very large nucleus have a greater change of flying apart? The STRONG force is a short range force, so a large nucleus may vibrate in such a way as to have part of it extend beyond the range of the strong force, and then electrostatic repulsion may cause a splitting of the nucleus. (Quantum mechanical “tunneling” may also be thought of as allowing such decays to occur.) 5. When thorium (atomic number 90) decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? Alpha decay always results in 2 protons and 2 neutrons (an alpha particle) being emitted, so the atomic number of the resulting nucleus must be 88 (radium). 6. What change in atomic number occurs when a nucleus emits an alpha particle? -2 A beta particle? +1 A gamma ray? 0 7. What is the long-range fate of all the uranium that exists in the world? It will continue undergoing radioactive decay so that there will be less and less of it as time goes by. 8. Why is the quantity of C-14 in new bones greater than in old bones of the same mass? The new bones just recently stopped being formed and taking in C-14 from their environment, so they will have a similar percentage of C-14 as the atmosphere. Old bones have taken in no new C-14 for a long time, so the radioactive C-14 has had a chance to decay into nitrogen, therefore lowering the quantity of C-14. 9. Where does most of the radiation you encounter originate? Either from space (the Sun) or from naturally radioactive rocks around us. 10. X-rays are most similar to which of the following – alpha, beta or gamma rays? Gamma rays, since they are also electromagnetic waves. 11. Gamma radiation is fundamentally different from alpha and beta radiation. What is this basic difference? Gamma rays are a form of light, and alpha and beta particles are massive particles, not EM waves. 12. Why are alpha and beta rays deflected in opposite directions in a magnetic field? Why are gamma rays not deflected? Differently electrically charged particles bend different directions in magnetic fields. Gamma rays have no charge, so they are not deflected at all. Page 1 of 2 NUCLEUS & RADIOACTIVITY QUESTIONS and ANSWERS 13. The alpha particle has twice the electric charge of the beta particle but, for the same kinetic energy, deflects less than the beta in a magnetic field. Why is this so? An alpha particle has about 8000 times the mass of a beta particle, so it is (relatively) very hard to accelerate the alpha particle and it does not deflect as much as a beta particle. 14. Which type of radiation – alpha, beta, or gamma – produces the least change in mass number? Gamma and beta (no change). In atomic number? Gamma (no change). 15. Just after an alpha particle leaves the nucleus, would you expect it to speed up? Defend your answer. Yes. It will feel a repulsive electric force acting on it from the nucleus. 16. Can it be truthfully said that, whenever a nucleus emits an alpha or beta particle, it necessarily becomes the nucleus of another element? Yes! 17. When food is irradiated with gamma rays from a cobalt-60 source, does the food become radioactive? Defend your answer. No! The gamma rays deposit energy in the food (or bacteria) but do not make the food or bacteria emit alpha, beta or gamma radiation. 18. The age of the Dead Sea Scrolls was found by carbon dating. Could this technique apply if they were carved in stone tablets? Explain. No! Carbon dating can only work with material that was once living and taking in some form of carbon. If the “Ten Commandments” were ever found, they would have to be dated by another technique. Page 2 of 2