Answers
advertisement

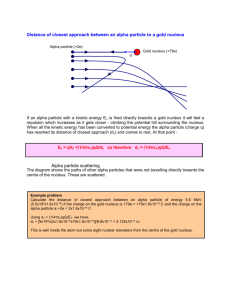
Day 8.2 Scattering – Calculations (7.6) 1) Rutherford used the following data to calculate the closest approach of an alpha particle to a gold nucleus. The masses, charges and speed of the particles are; qAu = 79×(1.6×10−19) C, q = 2×(1.6×10−19) C , m = 4 x 1.67×10−27 kg, v = 2.0 ×107 m/s, Use conservation of energy to calculate how close the alpha particle can get to the gold nucleus. Substituting these values into the equations for kinetic energy (1/2 mv2) and potential energy (k qau q /r), gives a value of closest approach of r = ½ m v2/ k qau q = 27×10−15 m. 2) In the calculation above, the effects of friction and air resistance A) are exactly zero B) are negligible C) cancel D) too hard to calculate In between the gold nuclei there is no air, so there is no air resistance. Friction is a result of electrostatic forces of many atoms in a solid acting upon many atoms in another solid. This experiment is set up so we can examine the effect of electrostatic forces between individual particles. There is no friction. 3) In the calculation above, we used classical - not relativistic - equations. What is gamma for a speed of 2 x 107 m/s? Was this a reasonable assumption? The alpha particle’s speed is 7% of the speed of light. Gamma is 1.002, a 0.2% error which is an error in the fourth digit. We are doing calculations to just two digits so it is irrelevant. 4) The calculation gives a maximum size. It is probably smaller than this. Why? How could you test this? The alpha particle’s approach distance is limited by its kinetic energy. Once all its kinetic energy has been transformed into potential energy it will be stopped and then start to repel back out. You need faster particles to test this. 5) The accepted value of the radius of the gold nucleus is 7.3×10−15 m. Make a sketch of the nucleus and the path of the alpha particle showing the relative sizes of the radius and closest approach. Calculate how fast an alpha particle would have to move to get this close. You can calculate the speed by plugging all the numbers in, or you can use proportions, v2 r = 2kQq/m. The radius has been reduced by 7.3/27, so the speed squared is increased by 27/7.3. Therefore, speed = 2.0 ×107 m/s x 1.9 = 3.8 ×107 m/s. 6) Rutherford repeated his experiment with aluminum foil and got a closest approach of 4.86 x 10-15 m. The accepted value of the nuclear radius for aluminum is 3.6 and x 10-15 m. Why are these two values closer to each other than those of the gold nucleus? a) the nuclear charge is only 13 e b) the nuclear mass is only 27 u c) both made it smaller d) something else The smaller charge results in a repulsive force that is smaller and the alpha particle can get closer before it stops. The force from an aluminum nucleus is 13/79 or 16% that of a gold nucleus. The smaller mass of the nucleus would make it harder, because the nucleus would move more. However, these nuclei are not free particles. They are held in a crystal structure and essentially immovable. Rutherford finally got an 180o scattering with aluminum, something he was never able to do with the gold. This scattering has the alpha particles get as close as possible for this speed. 7) In order to measure the actual nuclear radius, we need to get closer and therefore we need to have faster alpha particles. Rutherford was limited by the energy produced by radioactive alpha decay ~7.7 MeV. He encouraged physicists to build artificial accelerators. The graph below shows what happened when artificially accelerated alpha particles were sent toward lead nuclei (N = 208, Z = 82). a) Why does the graph change suddenly when the particles have energies greater than 27.5MeV? b) Calculate the radius of the lead nucleus. The alpha particles are hitting the nucleus of the lead. This is no longer simple Coulomb scattering, which is shown by the dotted curve. The nuclear forces are affecting the alpha particle. When we modeled the experiment with wine glasses, we could hear a noise when the ‘nucleus’ was struck. This is how you can tell if you hit the nucleus. If you did careful measurements you would also see a change in behaviour similar to the graph. r = kQq/E =9.00x109 x2ex82e/(27x 106 e) = 8.60 x 10-15 m Textbook: p. 625 # 4, 5 p. 371 # 3, 4 p. 380 # 34 435 #9