Fumaric Acid Synthesis: Chemistry Practical Manual
advertisement

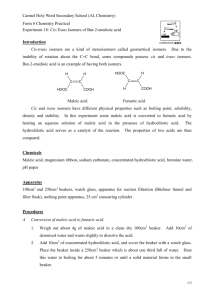
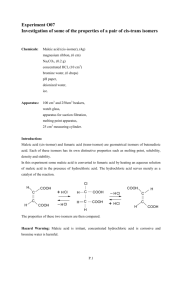
OUR LADY OF THE ROSARY COLLEGE CHEMISTRY PRACTICAL MANUAL Advanced Level, F.7 Experiment should be carried out under the supervision of a Chemistry teacher Safety precaution must be noted. Division of work: individual Preparation of Fumaric Acid from Maleic Acid Introduction Maleic acid and fumaric acid have the same molecular formula C4H4O4. Each contains two carboxylic acid groups and each exhibits the properties of an unsaturated organic compound with a C=C bond. However, they are geometric isomers. In this experiment you will convert some maleic acid to fumaric acid by heating in an aqueous solution containing some hydrochloric acid. The hydrochloric acid is not used up by the reaction but serves merely as a catalyst. Chemicals: Maleic acid (4 g), distilled water, concentrated hydrochloric acid Additional Apparatus for the class: 10-ml measuring cylinder, 400 cm3 beaker, triple beam balance, oven kept at 100 ℃, set of vacuum flask, suctional pump and Buchner funnel for suctional filtration. Procedure 1. 2. 3. 4. Weigh about 4 g of maleic acid in a 100 cm3 beaker. By using a measuring cylinder add 10 cm3 of distilled water and warm slightly to dissolve the acid. Add 10 cm3 of concentrated HCl, and cover the beaker with a watch glass. Place the beaker inside a 400 cm 3 beaker which is about 1/3 full of water. Heat this water bath to boiling for about 5 minutes or until a solid material forms in the small beaker. Cool the solution to room temperature by placing the small beaker with its contents in a cold water bath, or cool by ice. Filter the reaction mixture by suction, or in usual way (folded filtered paper). Wash any solid from the beaker onto the filter paper. 5. Transfer the dried crystals into a weighed watch glass. Dry the crystals by placing the solid in an oven at about 100 ℃ for 10 minutes. 6. When the sample is dry, weigh it on the watch glass. Label the sample "Fumaric acid" Data and results (A) Weighing maleic acid Mass of maleic acid and paper / g : Mass of paper / g: Mass of maleic acid / g: ____________ ____________ ____________ (B) Weighing fumaric acid Mass of fumaric acid and paper / g : Mass of paper / g: Mass of fumaric acid / g: ____________ ____________ ____________ Questions for discussion 1. 2. 3. 4. 5. 6. C=C bond cannot rotate freely. Explain. Maleic acid and fumaric acid have the same molecular formula, C4H4O4. Each contains two carboxyl groups and each exhibits the properties of an unsaturated organic compound with a C == C bond. Based on the above information, propose two structures, one of which could be maleic acid and the other is fumaric acid (You don't have to identify them.) Hydrochloric acid is added as a catalyst. Propose a reaction mechanism for the conversion of either(a) a cis-isomer to a trans-isomer or (b) a trans-isomer to a cis-isomer. Assuming the equilibrium concentrations were achieved in procedure A, which would you classify as the more stable with respect to transformation of one into the other? Explain the purpose of heating the reaction mixture in step 2. What is the purpose of cooling the solution in step 3? Draw a schematic diagram for the drying of fumaric acid by using a suctional pump.