Development of Modern Atomic Theory
advertisement

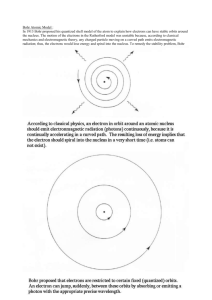
Development of Modern Atomic Theory 440 BC Continuous Theory of Matter: (Aristotle, Plato, et al.) a) matter can be divided and subdivided into smaller and smaller pieces without limit. Discontinuous Theory of Matter: (Democritus) a) matter was discontinuous, like a clump of grapes, it could be divided in half repeatedly until eventually only one grape was left that could not be divided. Democritus called this smallest particle of matter an atom (from the Greek atomos, meaning indivisible). This is the first historical mention of the atom. The major points of Democritus’ Discontinuous Theory: 1. All matter is composed of atoms, which are bits of matter too small to be seen. These atoms cannot be further split into smaller portions. 2. There is a void, which is empty space between atoms. 3. Atoms are completely solid. 4. Atoms are homogeneous, with no internal structure. 5. Atoms are different in their sizes and their shapes (and their weight, later added by Epicurus) The idea of the atom was strongly opposed by Aristotle and most others and would not reappear until the 18th century nearly 2000 years later. 1260 Roger Bacon (1214-1294) - 13th century English philosopher and scientist. Bacon was the first to formulate and advocate the use of the scientific method. 1400 - 1650 Alchemists - the first chemists who tried to turn base metals into gold, find a universal solvent, and find the elixir of life. Despite short comings (secrecy, mysticism, and outright fraud), they developed many experimental methods and built an extensive body of chemical data. 1744 Antoine Lavoisier - Law of the Conservation of Matter (Mass): states that matter can not be created or destroyed in ordinary chemical or physical changes. 1799 Joseph Proust - Law of Definite Composition/Proportions: a chemical compound contains the same elements in exactly the same proportions (ratios) by mass regardless of the size of the sample or source of the compound. 1803 Dalton - The Law of Multiple Proportions: “when two elements can be combined to make two different compounds, and if samples of these two compounds are taken so that the masses of one of the elements in the two compounds are the same, then the ratio of the masses of the other element in these compounds will be a ratio of small whole numbers. CO and CO2 NO3 and N2O3, NO3 and N2O2 FeCl2 and FeCl3 N2O3 and N2O4, 1808 Dalton's Atomic Theory – Based on the Laws of Conservation of Mass, Definite Composition, and Multiple proportions 1. All matter is composed of extremely small particles called atoms. 2. Atoms of a give element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. 3. Atoms cannot be subdivided, created, or destroyed. 4. Atoms of different elements can combine in simple, whole number ratios to form chemical compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged. a) Combination (Synthesis): b) Separation (Decomposition): c) Rearrangement: A + B AB AB A + B AB + CD AD + CB 1877 William Crookes – provided the first evidence of the existence of electrons using “Crookes Tube” (vacuum tubes). He called the particles/radiation he observed cathode rays. 1897 Thomson – discovers the electron using a modified Crookes Tube and calculates the charge to mass ratio (charge/mass) of the electron. Thomson concluded that cathode rays consisted of negatively charged particles which he called electrons. 1900 Max Planck – Planck found that light emitted by solids heated to various temperatures, was the same color and proposed that light has particle-like properties (i.e. can behave like matter) because it is composed of individual “bundles” of energy called quanta; objects radiate energy in small specific amounts (quanta). The energy of one quanta of radiation is given by E = h, where: E = energy, h = 6.63 x 10-34 J∙s (Planck’s constant), and “” is the frequency of the radiation. 1905 Einstein – building on Planck’s theory described light as having particle like properties; explains the photoelectric effect (electrons ejected from the surface of certain metals exposed to light of a minimum energy/frequency; describes a photon as a quantum of light (particles of radiation). Radiation is emitted or absorbed only in whole numbers of photons. 1909 Millikan calculates the charge of an electron using his oil drop experiment then determines an electron’s mass using Thomson’s charge/mass ratio. 1911 Rutherford discovers the nucleus using his gold foil experiment. Positively charged nucleus surrounded by mostly empty space. Electrons move around the nucleus. 1911 Henry Moseley – Discovered that the positive charge of the nucleus increases by one unit from one element to the next. He proposes that the elements in the periodic table be arranged in order of increasing atomic number instead of atomic mass. 1913 Bohr “Planetary Model” makes the connection between the wavelength of light an element emits and its atomic structure. Bohr’s model was based upon the work done by Planck and Einstein. Summary of Bohr’s Theory: Electrons travel in certain fixed orbits (each orbit having an associated energy) around the nucleus. Electrons are treated as particles and energy is quantized. An orbit is a place of fixed energy where an electron can be found; similar to the orbits of the planets around the sun. Orbits closest to the nucleus have the lowest energy. Bohr’s model of the atom worked well for the hydrogen but not for any others. Bohr’s Model 1. Electrons move around the atom’s nucleus in circular paths called orbits. 2. These orbits are definite distances from the nucleus and represent energy levels that determine the energies of these electrons. Electrons can not exist in any position outside these designated energy levels. (1, 2, 3,.. or E1, E2, E3,.. or K, L, M, N,…) 3. Electrons orbiting closest to the nucleus have the lowest energy; those farthest from the nucleus have the highest energies. 4. If electrons absorb energy, they move to higher energy levels; when they drop down to lower energy levels they release energy (as light). This energy (electromagnetic radiation) is seen as spectral lines and is given by E = h and since c = then E = hc/ where: h = 6.63 x 10-34 Js (Planck’s constant) 5. Energy is absorbed and given off in definite amounts called quanta which depends on the energy levels involved E = h where: E = Ehigher (final) – Elower (initial) = h 1919 Rutherford discovers the proton. 1924 DeBroglie proposes the concept of matter waves. If waves (light) can have particle-like properties as proposed by Planck and Einstein, then maybe matter can have wave like properties 1926 Schrodinger develops his wave equations using DeBroglie’s theory. He treats electrons moving around the nucleus as waves. His model applies to all atoms unlike Bohr’s model for hydrogen. This Quantum Mechanical Model replaces two dimension orbits with threedimensional orbitals. Quantum Mechanical or Wave Mechanical Model: 1. The electron is treated mathematically as a wave in the quantum mechanical model but as a particle in the Bohr model. The electron has properties of both particles and waves. 2. In the Bohr model, the energy of the electron is described in terms of a definite orbit. In the quantum mechanical model the energy is described in terms of the probability of locating the electron in a region of space outside the nucleus. The electron can be very close to the nucleus or very far away. However the probability of the electron being a certain distance from the nucleus most of the time is high 90% (0.529 A for the hydrogen 1s electron). A = 10-10 m (angtstrom) 3. In the quantum mechanical model, energy levels are not considered to be fixed orbits at specific distances from the nucleus. Instead, the energy levels are thought of as clouds surrounding the nucleus. 1928 Heisenberg develops his uncertainty principle; “it is not possible to know both the velocity and the position of a particle at the same time.” 1932 James Chadwick discovers the neutron.