part2electroncbrittanyf
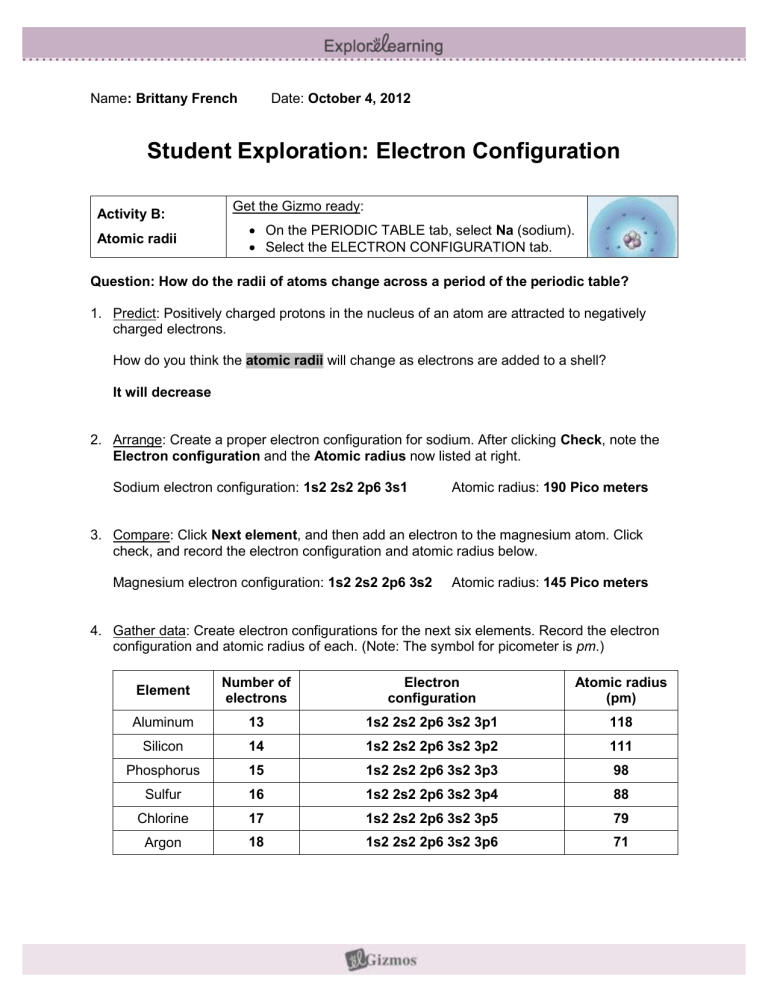
Name : Brittany French Date: October 4, 2012
Student Exploration: Electron Configuration
Get the Gizmo ready:
Activity B:
Atomic radii
On the PERIODIC TABLE tab, select Na (sodium).
Select the ELECTRON CONFIGURATION tab.
Question: How do the radii of atoms change across a period of the periodic table?
1. Predict: Positively charged protons in the nucleus of an atom are attracted to negatively charged electrons.
How do you think the atomic radii will change as electrons are added to a shell?
It will decrease
2. Arrange: Create a proper electron configuration for sodium. After clicking Check , note the
Electron configuration and the Atomic radius now listed at right.
Sodium electron configuration: 1s2 2s2 2p6 3s1 Atomic radius: 190 Pico meters
3. Compare: Click Next element , and then add an electron to the magnesium atom. Click check, and record the electron configuration and atomic radius below.
Magnesium electron configuration: 1s2 2s2 2p6 3s2 Atomic radius: 145 Pico meters
4. Gather data: Create electron configurations for the next six elements. Record the electron configuration and atomic radius of each. (Note: The symbol for picometer is pm .)
Element
Number of electrons
Electron configuration
Atomic radius
(pm)
Aluminum
Silicon
Phosphorus
Sulfur
Chlorine
Argon
13
14
15
16
17
18
1s2 2s2 2p6 3s2 3p1
1s2 2s2 2p6 3s2 3p2
1s2 2s2 2p6 3s2 3p3
1s2 2s2 2p6 3s2 3p4
1s2 2s2 2p6 3s2 3p5
1s2 2s2 2p6 3s2 3p6
118
111
98
88
79
71
5. Analyze: How does the atomic radius change across a period of the periodic table?
The atomic radius changes by it decreasing the bigger the atom and the larger number of electrons.
6. Interpret: Select the ATOMIC RADIUS tab. What do you notice? All of the elements are close together and they are all moving down the larger they are and the smaller the radius is.
7. Predict: On the ATOMIC RADIUS tab click Clear . Select the PERIODIC TABLE tab.
Elements in the same column of the periodic table are called chemical families , or groups.
How do you think the size of atoms will change from top to bottom within a chemical family?
They will get larger, because they are going from top to bottom on the periodic table.
8. Test: Hydrogen, lithium, and sodium are all in the same chemical family. Use the Gizmo to find the atomic radius of each, and list them below.
Hydrogen radius: 53 Lithium radius: 167 Sodium radius: 190
9. Analyze: How does the atomic radius change as you go from the top to the bottom of a chemical family? It gets larger going from top to bottom.
10. Challenge: Think about the factors that control atomic radius and the patterns you’ve seen.
A. Why does the atomic radius decrease as electrons are added to a shell? Because, they are attracting one another pulling them close, this would cause it to decrease.
B. Why does the atomic radius increase as you go from the top to the bottom of a chemical family? The atomic radius increases as you go from the top to bottom because they are adding energy levels, to hold more electrons.