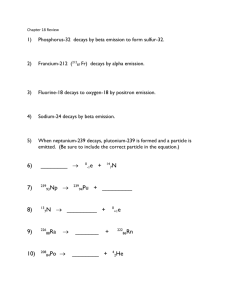
Independent Practice: Nuclear Reaction
advertisement
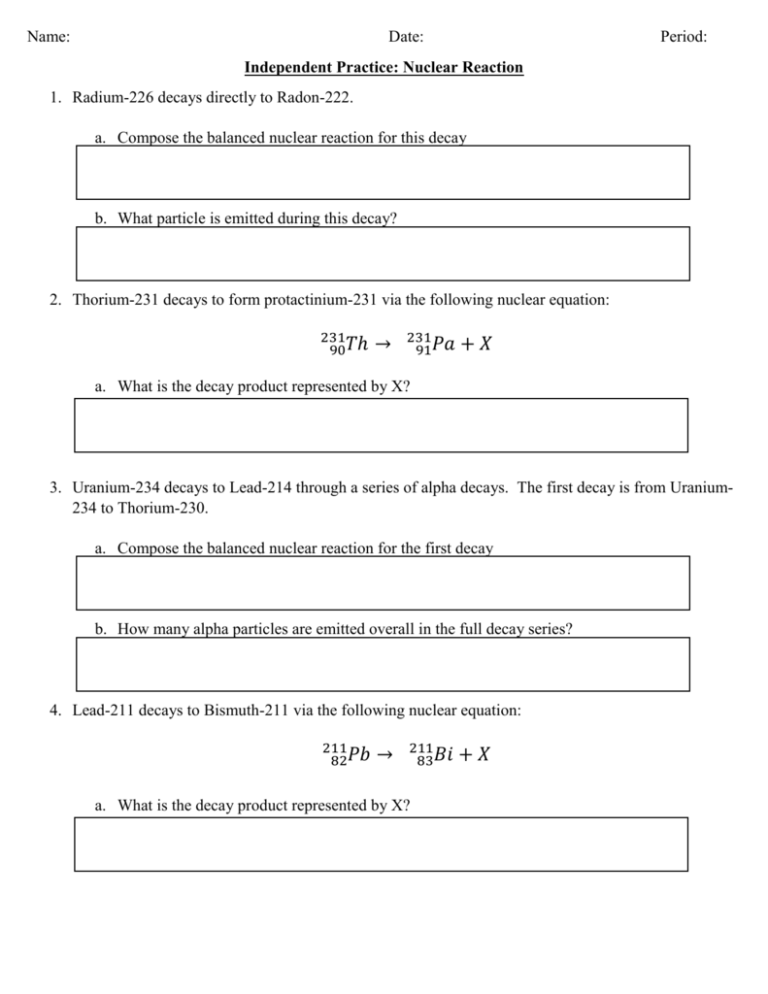
Name: Date: Period: Independent Practice: Nuclear Reaction 1. Radium-226 decays directly to Radon-222. a. Compose the balanced nuclear reaction for this decay b. What particle is emitted during this decay? 2. Thorium-231 decays to form protactinium-231 via the following nuclear equation: 231 90𝑇ℎ → 231 91𝑃𝑎 +𝑋 a. What is the decay product represented by X? 3. Uranium-234 decays to Lead-214 through a series of alpha decays. The first decay is from Uranium234 to Thorium-230. a. Compose the balanced nuclear reaction for the first decay b. How many alpha particles are emitted overall in the full decay series? 4. Lead-211 decays to Bismuth-211 via the following nuclear equation: 211 82𝑃𝑏 → 211 83𝐵𝑖 a. What is the decay product represented by X? +𝑋 5. The equation below shows the radioactive decay of Plutonium (Pu): 241 94𝑃𝑢 241 95𝐴𝑚 → + Radiation a. What particle is released in this reaction? 6. Write balanced equations for the following elements that are involved in alpha decay reactions. a. 212 84𝑃𝑜 → b. 237 93𝑁𝑝 → c. 207 83𝐵𝑖 d. → _______________ → 214 84𝑃𝑜 + ____________ 7. Write balanced equations for the following elements that are involved in beta decay reactions. a. 47 21𝑆𝑐 → b. 144 59𝑃𝑟 c. 14 6𝐶 d. → → _______________ → 65 30𝑍𝑛 + ___________