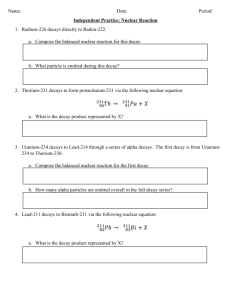
Chemistry Chapter 25 Study Sheet I. Practice Problems 1.) An 90.0
advertisement

Chemistry Chapter 25 Study Sheet 1 I. Practice Problems (amount remaining) = (initial amount) 2 n t 1 T (amount remaining) = (initial amount) 2 1.) An 90.0-g sample of a radioactive compound, thorium-234, is reduced to 56.7 g in 16 days. What is the half-life of the compound? 2.) An 100.0-g sample of a radioactive compound, uranium-238, is reduced to 83.1 g in 1.2 billion years. What is the half-life of the compound? 3.) A radioisotope of pottasium-32 having half-life of 22.0 hours, is administered to a patient for treatment of kidney cancer. Calculate the time by which 50.0 micrograms falls to 18.00% of the initial value in the body. 4.) A radioisotope of strontium-90 having half-life of 28.0 years, is administered to a patient for treatment of bone cancer. Calculate the time by which 10.0 micrograms falls to 10.00% of the initial value in the body. 5.) A radioactive compound uranium-234 has a half-life of 247000.0 years. What will be the amount remaining in a 500.0-g sample after 14700.0 years? 6.) A radioactive compound lead-210 has a half-life of 22.0 years. What will be the amount remaining in a 400.0-g sample after 10.0 years? II. Information 1. Wilhelm Roentgen discovered X-rays when he found that invisible rays were emitted when electrons bombarded the surface of certain materials as evidenced by the darkening of photographic plates. 2. Henri Becquerel accidentally discovered that phosphorescent uranium salts produced spontaneous emissions that darkened photographic plates. 3. Marie & Pierre Curie named the process by which materials give off rays and particles radioactivity; they named the rays and particles emitted by a radioactive source radiation. They also discovered the elements Po & Ra. 4. Ernest Rutherford identified alpha, beta, and gamma radiation when studying the effects of an electric field on the emissions from a radioactive source. He was also the first to perform induced transmutation when he bombarded N-14 with high-speed alpha particles. 5. Know the information in these charts. Characteristics of Chemical & Nuclear Reactions Chemical Reactions Nuclear Reactions 1. Occur when bonds are broken and formed. 1. Occur when nuclei emit particles and/or rays. 2. Atoms remain unchanged, through they may be rearranged. 2. Atoms are often converted into atoms of another element. 3. Involve only valence electrons. 3. May involve protons, neutrons, & electrons. 4. Associated with small energy changes. 4. Associated with large energy changes. 5. Reaction rate is influenced by temperature, pressure, concentration, & catalysts 6. Reaction rate is not normally affected by temperature, pressure, or catalysts. Property Composition Notation Charge Approximate energy Relative penetrating power Properties of Alpha, Beta, and Gamma Radiation Alpha Beta alpha particles beta particles 4 2 He +2 5 MeV blocked by paper 0 1 -1 0.005 to 1 MeV blocked by metal foil Gamma high-energy electromagnetic radiation 0 0 0 1 MeV not completely blocked by lead or concrete Type of decay Alpha decay Summary of Radioactive Decay Processes Particle emitted Change in mass # 4 -4 He 2 0 +1 0 -1 X-ray photon 0 0 -1 0 Beta decay 0 1 Positron emission 0 1 Electron capture Gamma emission Change in atomic # -2 0 0 6. Radioisotopes decay in order to reduce the number of neutrons or protons to lie in the band of stability. The band of stability is a graphical representation of a neutron-to-proton ratio between 1 : 1 and 1.51 : 1. 7. Be able to predict the type of decay: (1) If the atomic # is greater than 83, it will undergo alpha decay. If the atomic # is 83 or less… (2) Calculate the neutron-to-proton ratio of the isotope listed on the periodic table and the given isotope. If they are close, it won’t decay. If the given isotope’s ratio is… (3) …significantly higher, it will undergo beta decay. (4) …significantly lower, it will undergo positron emission or electron capture. 8. Just a reminder: atomic number tells the number of protons; to calculate neutrons, subtract the atomic number from the mass number. And the number following a chemical symbol (like C-14) tells you the mass number. Neutron-to-proton ratio is neutrons divided by protons to 1 9. Things to remember when writing & balancing nuclear equations Think of the arrow as an equal sign. The sum of the top numbers (masses) must be equal on both sides. The same goes for the sum of the bottom numbers (atomic numbers). “Bombarded with” means that thing goes on the left side of the equation. “Emits” or “emission” or “decays by” or “products” means that thing goes on the right side of the equation. “Capture” means that thing goes on the left side of the equation. If the question doesn’t tell you what type of decay is going on, you must determine that first using the guidelines you’ve been given previously. 10. Practice nuclear equation problems 1.) Write a balanced nuclear equation for the decay of thorium-230. 2.) Write a balanced nuclear equation for the decay of radium-226. 3.) Write a balanced nuclear equation for the reaction of oxygen-15 undergoing positron emission. 4.) Write a balanced nuclear equation for electron capture by krypton-76. 5.) Write a balanced nuclear equation for the bombardment of einsteinium-253 by an alpha particle. One of the products is a neutron. 6.) Write a balanced nuclear equation for electron capture by silver-106. 7.) Write a balanced nuclear equation for the bombardment of beryllium-9 by an alpha particle. 8.) Write a balanced nuclear equation for the reaction of potassium-38 undergoing positron emission. Answers I. Practice Problems 1.) 24.0 days 2.) 4.49 billion years 3.) 54.4 hours 4.) 93.0 years 5.) 479.8 g 6.) 291.9 g