The mole
advertisement
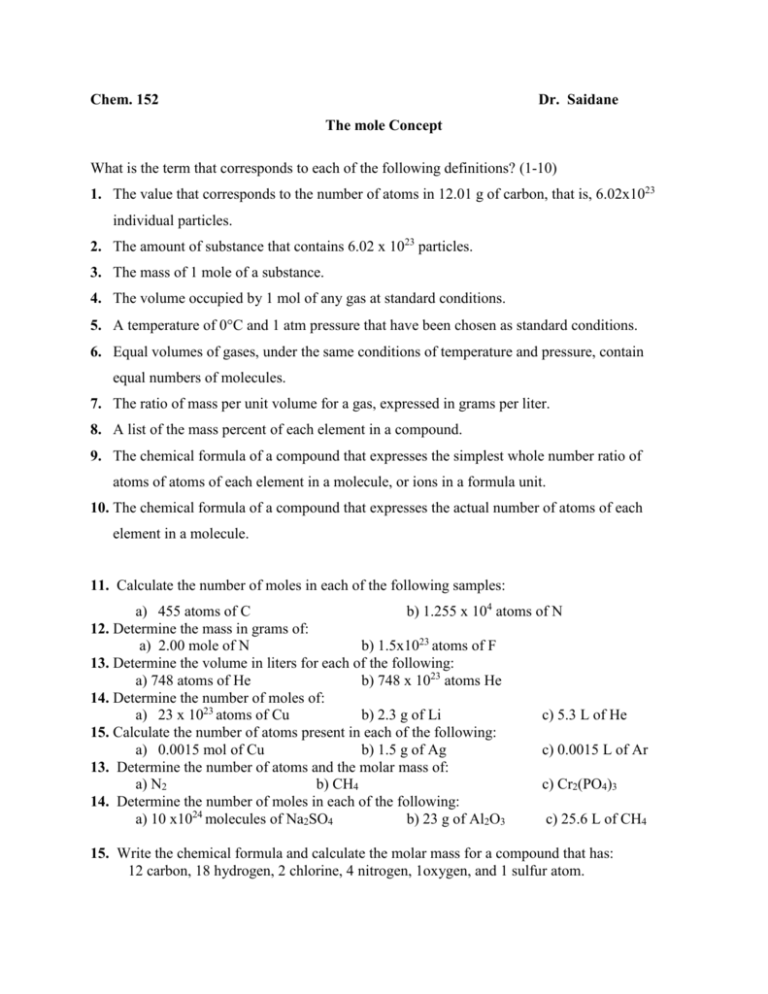
Chem. 152 Dr. Saidane The mole Concept What is the term that corresponds to each of the following definitions? (1-10) 1. The value that corresponds to the number of atoms in 12.01 g of carbon, that is, 6.02x1023 individual particles. 2. The amount of substance that contains 6.02 x 1023 particles. 3. The mass of 1 mole of a substance. 4. The volume occupied by 1 mol of any gas at standard conditions. 5. A temperature of 0C and 1 atm pressure that have been chosen as standard conditions. 6. Equal volumes of gases, under the same conditions of temperature and pressure, contain equal numbers of molecules. 7. The ratio of mass per unit volume for a gas, expressed in grams per liter. 8. A list of the mass percent of each element in a compound. 9. The chemical formula of a compound that expresses the simplest whole number ratio of atoms of atoms of each element in a molecule, or ions in a formula unit. 10. The chemical formula of a compound that expresses the actual number of atoms of each element in a molecule. 11. Calculate the number of moles in each of the following samples: a) 455 atoms of C b) 1.255 x 104 atoms of N 12. Determine the mass in grams of: a) 2.00 mole of N b) 1.5x1023 atoms of F 13. Determine the volume in liters for each of the following: a) 748 atoms of He b) 748 x 1023 atoms He 14. Determine the number of moles of: a) 23 x 1023 atoms of Cu b) 2.3 g of Li c) 5.3 L of He 15. Calculate the number of atoms present in each of the following: a) 0.0015 mol of Cu b) 1.5 g of Ag c) 0.0015 L of Ar 13. Determine the number of atoms and the molar mass of: a) N2 b) CH4 c) Cr2(PO4)3 14. Determine the number of moles in each of the following: a) 10 x1024 molecules of Na2SO4 b) 23 g of Al2O3 c) 25.6 L of CH4 15. Write the chemical formula and calculate the molar mass for a compound that has: 12 carbon, 18 hydrogen, 2 chlorine, 4 nitrogen, 1oxygen, and 1 sulfur atom. 16. Complete the following table: Gas Molecules CO2 7.48 x 1020 CO O2 Mass Volume at STP 7.48 g 7.48 L 17. Find the percentage composition of Lead(II) chloride and Barium nitrate. 18. Magnesium hydroxide is 54.87% oxygen by mass. How many grams of oxygen are in 175 g of the compound? How many moles of oxygen is this? 19. Find the empirical formula of a compound that contains 26.56% potassium, 35.41% chromium, and the remainder oxygen. 20. Analysis of 20.0 g of a compound containing only calcium and bromine indicates that 4.00 g of calcium are present. What is the empirical formula of the compound formed?











