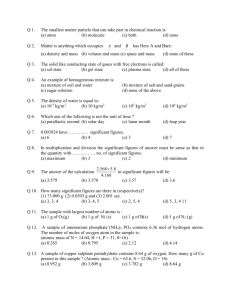
maitland/5231/P5Quantitative Aspects of Formulae and Equations
advertisement
P5 Quantitative Aspects of Formulae and Equations The mole concept is one of the fundamental concepts of modern chemistry as the use of this concept enables scientists and science students to investigate the quantitative relationships in chemical reactions. Formulae and stoichiometric equations are used in these calculations. Stoichiometry The study of quantitative aspects of formulae and equations Relative atomic mass The average mass of the atoms present in the naturally occurring element relative to the mass of an atom of the carbon-12 isotope Commonly called atomic weight Not the mass of an atom of that element, it is a ratio and has no units. Relative molecular mass The mass of a molecule of the compound relative to the mass of an atom of the carbon-12 isotope The sum of the atomic weights of the atoms as given in the molecular formula Relative formula mass The sum of the atomic weights of the atomic species in the stated formula of the compound The empirical formula is used for ionic compounds Mole Avogadro’s number of particles (6.02 x 1023) The quantity of a substance that contains as many atoms, ions or molecules as there are atoms in exactly 12 grams of the carbon-12 isotope Mole of an element The mass which is numerically equal to the atomic weigh of the element Mole of a compound The mass which is numerically equal to the molecular weight of the compound Molar mass The mass of a mole of the substance Mole formulae Where n = m M n = number of moles m = mass in grams M = atomic or molecular weight in grams N = n x 6.02 x 1023 Where N = number of atoms or molecules n = number of moles 6.02 x 1023 is Avogadro’s number Percentage composition Mass of the element in one mole of the compound Mass of one mole of the compound Empirical formula Simplest whole number ratio of the atoms of each element in the compound Molecular formula The number of each type of atom in a molecule of the compound. Calculating an empirical formulae x 100 Write down the masses of all the elements present in a given sample of the compound Convert masses to moles by dividing each mass by its atomic weight Divide through by the smallest number of moles to get a simple ratio Multiply through by a suitable factor to get whole numbers Round off the numbers to get whole numbers and use these to write the empirical formulae Chemical equation Describes the ratio by mass in which substances react or are formed in a reaction. Performing a massmass calculation Write a balanced chemical equation Calculate the number of moles of the given substance Use the stoichiometric coefficients in the chemical equation to link the number of moles of the given substances to the number of moles of the required substance Use this link to calculate the number of moles of the required substance Calculate the mass of the required substance. Atomic theory This theory was proposed by John Dalton in 1803 and the four postulates were Matter is composed of small indivisible spheres (atoms) All atoms of one elements are identical The atoms of different elements are different Atoms of different elements combine in simple whole number ratios. Law of combining volumes Also known as Gay-Lussac’s Law When measured at constant temperature and pressure, the volumes of gases taking part in a chemical reaction show simple whole number ratios to one another Avogadro’s hypothesis When measured at the same temperature and pressure, equal volumes of gases contain the same number of molecules. When measured at the same temperature and pressure, equal numbers of gas molecules occupy the same volume Percentage yield The amount of product obtained from a chemical reaction expressed as a percentage of the amount expected from the chemical equation.