Atomic Physics Pastpaper Solution
advertisement
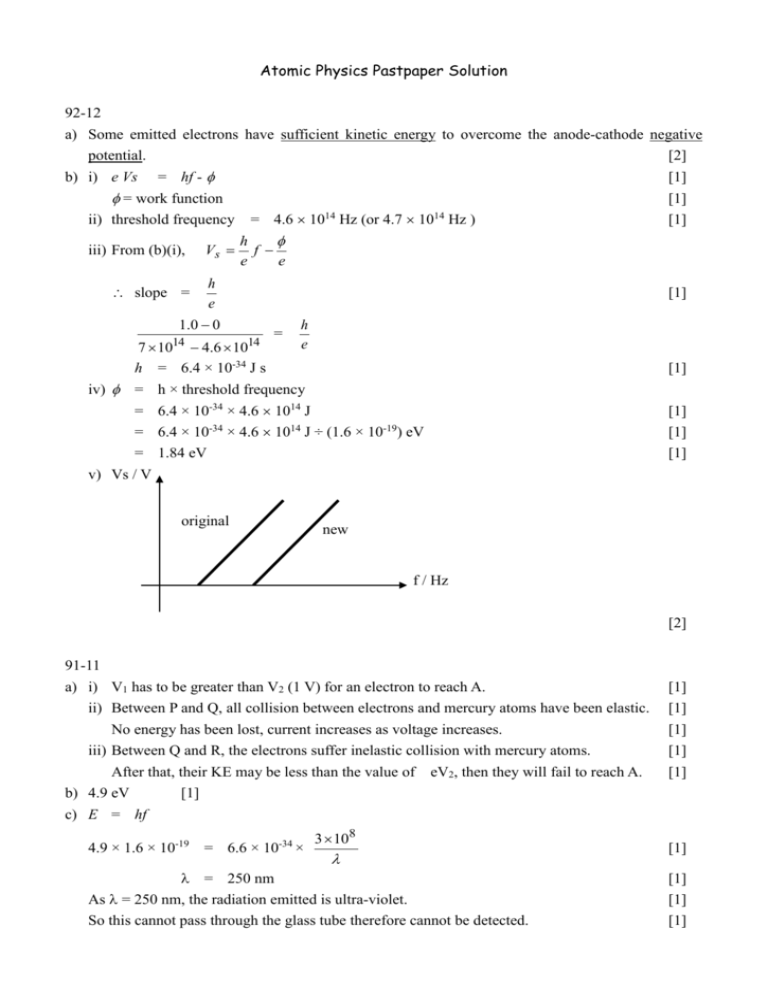
Atomic Physics Pastpaper Solution 92-12 a) Some emitted electrons have sufficient kinetic energy to overcome the anode-cathode negative potential. [2] b) i) e Vs = hf - = work function ii) threshold frequency iii) From (b)(i), Vs = 4.6 1014 Hz (or 4.7 1014 Hz ) h f e e h e 1.0 0 slope = 7 1014 4.6 1014 h = 6.4 × 10-34 J s iv) = = = = v) Vs / V [1] [1] [1] [1] = h e [1] h × threshold frequency 6.4 × 10-34 × 4.6 1014 J 6.4 × 10-34 × 4.6 1014 J ÷ (1.6 × 10-19) eV 1.84 eV original [1] [1] [1] new f / Hz [2] 91-11 a) i) V1 has to be greater than V2 (1 V) for an electron to reach A. ii) Between P and Q, all collision between electrons and mercury atoms have been elastic. No energy has been lost, current increases as voltage increases. iii) Between Q and R, the electrons suffer inelastic collision with mercury atoms. After that, their KE may be less than the value of eV2, then they will fail to reach A. b) 4.9 eV [1] c) E = hf 4.9 × 1.6 × 10-19 = 6.6 × 10-34 × 3 108 = 250 nm As = 250 nm, the radiation emitted is ultra-violet. So this cannot pass through the glass tube therefore cannot be detected. [1] [1] [1] [1] [1] [1] [1] [1] [1] d) i) Before the electrons reached the energy of 6.7 eV, [1] it has already had an inelastic collision (at an energy of 4.9 eV) with the mercury atoms. [1] so the energy of electrons never reaches 6.7 eV. [1] ii) As there is no energy interval corresponding to 9 eV, the photon will be scattered and will not be absorbed. [2] 96-9 a) The minimum energy/work one for liberating an electron from the surface of metal. [1] b) Part of the energy gained by an electron from a photon is lost through a number of collisions with the surrounding electrons/ions. [1] c) i) KEmax = hf - = 6.63 × 10-34 × 3 108 230 10 - 2.21 × 1.6 × 10-19 9 = 5.11 × 10-19 J (or 3.19 eV) ii) eVs Vs [1] [1] = KEmax = 5.11 10 19 1.6 10 19 = 3.19 V d) Energy supplied per second = 3 × (8.0 × 10-3)2 = 1.92 × 10-4 J Energy of each photon = 6.63 × 10-34 × [1] [1] 3 108 230 10 9 = 8.65 × 10-19 J No. of photoelectrons emitted per second = No. of photons absorbed = 1.92 × 10-4 J ÷ 8.65 × 10-19 J = 2.22 × 1014 e) Stopping potential would increase as the energy of each photon increases, the maximum KE of the photoelectrons emitted increases. The number of photoelectrons emitted per second would decreases since intensity is constant and each photon has more energy, the number of photons arrive per second decreases. [1] [1] [1] [1] [1] [1] 94--5 a) i) min. wavelength min = 1.2 × 10-11 m hc max. energy of X-ray photons = = (6.6 10 34 )(3 108 ) 1.2 10 11 [1] = 1.65 × 10-14 J ii) v = 1.65 10 14 1.6 10 19 = 103 kV iii) lower intensity same min , line positions b) n [1] = no. of electrons striking the target per second 600 = (1.65 10 14 )(0.995) = 3.65 × 1016 c) Continuous spectrum [1] [1] [1] [1] [1] Some electrons are stopped by the target and their KE is converted directly to X-rays. [1] The bombarding electron may be brought to rest in one or more collisions in which they give up energy gradually, thus emitted photons of different wavelengths. [1] Line Spectrum Some bombarding electrons have sufficient energy to dislodge the inner electrons of a target atom, Leaving a vacant space in the shell, as electrons of upper shells fill the vacancy, photons of definite frequencies are emitted. [2] d) X-rays are emitted when the high energy electrons from the cathode of the TV are stopped. [1] Method : reduce the accelerating potential [1]