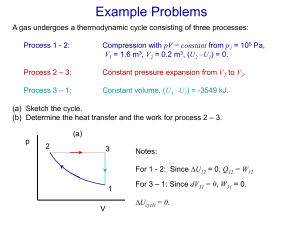
Lecture_9_Surface Th..
advertisement

Thermodynamics of surface and interfaces
Define
:
Consider to be a force / unit length of surface perimeter.
(fluid systems)
If a portion of the perimeter moves an infinitesimal of distance in the plane
of the surface of area A, the area change dA is a product of that portion of
perimeter and the length moved.
Work term - dA; force x distance, and appears in the combined 1st
and 2nd laws of thermodynamics as
dU TdS pdV
dN
i
i
i
dA
Strictly speaking , is defined as the change in internal energy when
the area is reversibly increased at constant S, V and Ni (i.e., closed
system).
For a system containing a plane surface this equation can be readily integrated :
U TS PV
N
i
i
A
i
and rearranging for yields.
1
U TS PV i N i
A
i
where U – TS + PV is the Gibbs free energy of the system, i.e., the actual
energy of the system
And
N
i
i
i
is the Gibbs free energy of the materials comprising the
system, i.e., the energy of the system as if it were
uniform ignoring any variations associated with the
surface
Thus is an excess free energy due to the presence of the surface.
def
Surface Excess Quantities
Macroscopic extensive properties of an interface separating bulk phases
are defined as a surface excess.
There is a hypothetical 2D “dividing surface” defined for which the
parameters of the bulk phases change discontinuously at the dividing
surface.
def
The excess is defined as the difference between the actual value of the
extensive quantity in the system and that which would have been
present in the same volume if the phases were homogeneous right up to
the “ Dividing Surface ” i.e.,
x x
s
total
The real value of x in
the system
x x
The values of x in the homogeneous
and phases
Concept of the Gibbs Dividing Surface
Extensive property
Density
Distance perpendicular to the surface
For a 1 component system the position of the dividing surface is chosen such
that the two shared areas in the figure are equal. This yields a consistent value
(equal to zero ) for the surface excess.
For a multicomponent system the position of the dividing surface that makes
some Ni equal to zero will be unlikely to make all the other Nj ≠i = 0.
By convention, N1, the surface excess of the component present in the largest
amount (i.e., the solvent) is made zero by appropriate choice of dividing
surface.
Alternatively if we consider a large homogeneous crystalline body containing
N atoms surrounded by plane surfaces then if U0 and S0 are the energy and
entropy / per atom, the surface energy per unit area Us is defined by
U N U AU N U A
0
s
0
where U is the total energy of the system.
:U
s
Similarly
S S N AS
0
s
Consider once again the combined form of 1st and 2nd laws including the
surface work term.
dU TdS pdV
dN
i
i
dA
i
Substitution of the definition of G leads to
dG SdT Vdp i dN i dA
i
If the surface is reversibly created in a closed system (Ni fixed) at constant T
and P.
G
A T ,P ,N i
is always the free energy change appropriate to the constraints imposed on
the system.
Since for the bulk phases and the surface terms vanish, the combined
1st and 2nd law take the form
dU
T dS
pdV
i dN
dN
i
i
and
dU
T dS
pdV
i
i
i
and for the total system
dU T dS pdV
dN
i
i
dA
i
From the definition of surface excess:
dU T dS pdV
s
s
s
0
i
X
s
X X X
i dN
s
i
dA
By Def.
Integration yields,
N
U TS
s
s
i
A
i
i
Forming the Gibbs-Duhem relation :
0 S dT N i d i Ad
s
i
so
d sdT
d
i
Gibbs-Adsorption Equation
i
i
where
s
S
s
s
A
;
i
Ni
A
Solid and liquid Surfaces
In a nn pair potential model of a solid, the surface free energy can be thought
of as the energy/ unit -area associated with bond breaking. :
work/ unit area to create new surface =
n
2
A
where n/A is the # of broken bonds / unit-area and the
is the energy per
bond i.e., the well depth in the pair-potential.
Then letting A =
a2
where a lattice spacing
2a
2
pair potential
If the solid is sketched such that
U(r)
the surface area is altered
a a da
A A dA
r
the energy
d
The total energy of the surface U S Asurf .is changed by an amount.
dU dA Ad
and
dU
dA
f A
d
dA
Surface Stress and Surface Energy
Unit Cube
W1=2
1
fxx
Split
Stretch
W2
w1
The difference in the work per
unit area required for the
constrained stretching (fix
dimension in the y direction while
stretching along the x-direction) is
defined as the surface stress, fxx.
This is the excess work owing to
the presence of the surfaces.
fxx
w2=2(+d)(1+dx)
1+dx
Shuttleworth cycle relating surface stress, f and surface energy, .
Surface Stress and Surface Energy
Relation between fij and
Consider 2 paths to get to the same final state of the deformed halves.
Path I - The cube is first stretched Path II - The cube is first separated
and then separated.
and then stretched.
WI = w1 + w2
WII = W1 W2
= w1 + 2(1+dx) ( D)
= 2 W2
= w1 + 2 2 D 2 dx
where Dxx (= dx/1) has caused a
change D in .
Since WI = WII,
w1 + 2 2 D 2 Dxx = 2 W2
work/unit area = (W2 - w1)/2Dxx = fxx = + D /Dxx
Surface stress, surface free energy and chemical equilibrium of small crystals
Recall that for finite-size liquid drops in equilibrium with the vapor.
(see condensation discussion)
l 0
2
r
Vl
Equil. cond.
where Vl is the molar vol. of the liquid.
For a finite-size solid of radius r the internal pressure is a function of the size
owing to the surface stress {isotropic surface stress}.
s 0
2f
r
Vs
The pressure difference between the finite-size solid in equil. with the liquid is
Ps Pl
2f
r
Consider the equilibrium between a solid sphere and a fluid containing the dissolved solid.
r
The total energy of the system is given by
dU TdS pdV
dN
i
dA
=0
i
d U T d S s ( so lid ) T d S l ( liq u id ) T d S ( su rfa ce ) p s d V s p l d V l p d V
N
+ dN dN
s
1
s
1
l
1
l
1
N
dN
s
i
i2
s
i
i dN i dA
l
l
i2
Gibbs dividing surface set for component 1, other components are not allowed
to cause area changes.
dU T dS s ( solid ) T dS l ( liquid ) T dS ( surface ) p s dV s p l dV l
+ 1 dN 1 1 dN 1 dA
s
s
l
l
Consider the variation dU = 0 under the indicated constraints,
dU 0;
dS s ( solid ) dS l ( liquid ) dS ( surface ) 0,
dV 0 dV S dV l ;
dV S dV l
dN 1 0 dN 1 dN 1 0;
S
dN 1 dN 1
l
S
l
Making the substitutions
dU ( p s p l ) dV s ( 1 1 ) dN 1 dA 0
s
l
s
and for a sphere, dA = (2/r)dVs
0 ( p s p l ) dV s ( ) dN
s
1
l
1
s
1
2
r
dV s
since dV s / dN 1 V s / N 1 V o the m olar volum e
s
s
2
( p s pl )
Vo
r
s
1
l
1
A lso since, p S p l
s
1
l
1
2f
r
2( f )
r
Vo
Now consider an N component solid of which components 1, ….. k are
substitutional and k +1, …. N are interstitial.
Note that the addition or removal of interstitial atoms leaves AL unchanged.
Then
N
il
N is U s TS s P 2 / r V s
is
N is U s TS s P 2 f / r V s
i 1
N
i 1
and
N
(
i 1
is
il ) N is 2 ( f )V s / r
For interstitial exchange : fluid --interstitial--- solid
dA L 0
is il ,
ⓐ
i k 1,....... N
For substitutional exchange : fluid -- substitutional --- solid
k
(
is
il ) N is 2 ( f )V s / r ,
i 1 .... k
i 1
k
and defining
N is N 0 and V s / N 0 as V 0 the molar volume.
i 1
ⓑ
is il 2 ( f )V 0 / r ,
i 1 .... k
Examples of how finite – size effects alter equilibria
(1) Vapor pressure of a single – component solid
l ( p e ) R T ln P / Pe
s ( p e ) 2V 0 f / r
V 0 ( P Pl )
0 in com parision to f term
using ⓑ
s l 2V 0 f / r RT ln p / p l 2 ( f )V 0 / r
RT ln p / p l 2 V 0 / r
same result as earlier
(2) Solubility of a sparingly soluble single component solid :
il i ( C e ) R T ln C / C e
is i ( C e ) 2V 0 f / r
using ⓑ
RT ln C / C l 2 V 0 / r
(3) Melting point of a single component solid :
l (Tm ) S l Tm T
s (T m ) S s T m T
where Sl and Ss are molar entropies.
2f
r
see
Clausius – Clapyron
Vo
Equation
using ⓑ
Tm
T
2 V0
r
1
Sl
Ss
2 V 0 Tm
r
Lf
, Sl Ss
Lf
Tm
(4) Vapor pressure of a dilute interstitial component in a non-volatile matrix
( H in Fe….)
If the interstitial vaporizes as a molecule:
nx x n
or if it reacts with a vapor species, A, forming a compound AmXn
mA nX Am X n
The chemical potential of X in the vapor is related to the partial pressure
P of Xn or AmXn by
xl p l
RT
ln
n
p
pl
and for the solid
xs p l 2V x f / r
when Vx is the molar volume of X in the solid.
Using ⓐ
RT ln p / p l 2 n V x f / r
indicating that f determines the change in vapor pressure