Lecture 3
advertisement

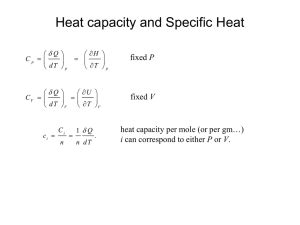
The Third E …. Lecture 3 Enthalpy But first… Uniting First & Second Laws • First Law: dU = dQ + dW • With substitution: dU ≤ TdS – PdV • For a reversible change: dUrev = TdSrev – PdV Defining Enthalpy • Enthalpy is the heat gained or lost by the system if the change takes place at constant pressure and P–V work is the only work done by the system dH = ∆QP • More formally: H = U + PV • Consequently: dH = dU +PdV +VdP • But recalling that dU ≤ TdS – PdV • What do we have left? Enthalpy and Heat dH ≤ TdS + VdP • For a reversible reaction: dHrev = TdSrev + VdP • And, if the (reversible) change takes place at constant pressure: dHP = TdSP = dQP Enthalpies of Reaction, Formation, Fusion, … • For an isobaric experiment in which: 2H2 + O2 = 2H2O o the enthalpy is simply the heat given off by the reaction. We can measure this in a calorimeter. o This is called a heat of reaction, ∆Hr o If the reactants are elements, it is the heat of formation: , ∆Hf • Another experiment we might conduct is a phase change, e.g., melting ice to form (liquid) water. o Enthalpy, in this case, is just the energy we would have to add to the ice to melt it. o Formally, this is called the latent heat of fusion; to avoid confusion, we will call it the heat of melting, ∆Hm (heat of crystallization is just the negative of this). o We can similarly define latent heat of evaporation (or condensation). Value of Enthalpy • It is usually easier to conduct experiments at constant pressure rather than constant volume. • These kinds of experiments (e.g., combusting hydrogen, melting ice) provide the basic data of thermodynamics that allow us ultimately to predict the outcome of natural reactions (like weathering of rock). We can calculate other thermodynamic properties from the enthalpy. Deriving Entropies • Since: • dHrev = TdSrev + VdP • If we conduct an experiment at constant pressure, then: dHrev = TdSrev • And if we also did the experiment at constant temperature, e.g., melting ice: ∆S = ∆H/T • If the reaction is not at constant temperature (melting of rock would not be), we would have to integrate: S=ò dH (T ) T Calorimetry • A calorimeter in simply a device in which we conduct a reaction, then measure the change in temperature in the vessel. • But, we need to know something additional: How much energy change does that temperature change correspond to? In other words, how much energy would we have to put into the system to change its temperature by 1 K? Heat Capacity • The thing we need to know the Heat Capacity. • We define it as: C = dQ/dT • At constant pressure: CP = (∂H/∂T)P • At constant volume since dU = dQ –PdV CV = (∂U/∂T)V • However, as we noted, experiments are usually not conducted at constant volume (except for gases), so mostly we will work with CP. Heat Capacities of Solids • Heat capacities of solids are complex functions of temperature as vibrational motions of atoms in solids is quantized. Theoretical calculation requires knowing all possible vibrational motions and energy levels. • In practice, heat capacities are empirically determined. In geochemistry, the 5-term Haas-Fisher formulation is generally used: c 2 -1/2 CP = a+bT - 2 + fT + gT T • Where a, b, c, ƒ, g are empirically determined constants. • We will use the simpler Maier-Kelley formulation: CP = a+bT - c2 T Boltzmann Distribution Law • While energy is on average shared equally among atoms or molecules of a substance, this does not mean that in detail they all have exactly the same energy. • Theoretical calculation of heat capacity requires knowing how energy is shared between atoms or molecules. • This is given by the Boltzmann Distribution Law, which states that the probability of an atom being in energy state εi is: e- ei /kT Pi = - e n /kT e å n • k is Boltzmann’s constant, the microscopic equivalent of the gas constant k = R/Av (where Av is Avagadro’s number) and the sum is over all possible energy states. Boltzmann’s Distribution Law In English: • Energy among atoms is like money among men: the poor are many and the rich few. Occupation of vibrational energy levels. The probability of an energy level associated with the vibrational quantum number n calculated from the Boltzmann distribution function of n for a hypothetical diatomic molecule at 273 K and 673 K. The Partition Function • The denominator of the Boltzmann Distribution Law is a key quantity in statistical mechanics called the Partition Function: Q = å e-en /kT n • Text explains how it is related to energy and entropy. • We will use it later in the course to calculate equilibrium constant and isotope fractionation factors. Relationship of Entropy to other State Variables æ ç ç è ¶S ö÷ = CV ¶T ÷øV T æ ç ç è æ ç ç è ¶S ö÷ = CP ¶T ÷ø P T ¶S ö÷ = -aV ¶P ÷ø T æ ç ç è ¶S ö÷ = a ¶V ÷ø T b Calculating Entropy and Enthalpy Changes • Since: o then æ ¶H ö çè ÷ø = CP ¶T V dH = CP dT • The enthalpy change of a substance heated from T1 to T2 is then: T2 ∆ H = ò CP dT T1 • For an entropy change it is: T2 C P dT T1 T ∆S=ò What if both Temperature and Pressure Change? • Entropy dependence on pressure is: æ ç ç è ¶S ö÷ = -aV ¶P ÷ø T P2 • So: ∆ S = òP -aVdP 1 o (for gases, V will be a function of P; we can sometimes assume solids are incompressible). • We need to do both the T and P integrals! • But, in a natural process, T and P will likely change simultaneously! o For example, a rising parcel of air or sinking parcel of seawater will experience simultaneous changes of P and T. • Does it matter? No! • For state functions, the result does not depend on the path taken. • Entropy and enthalpy are state functions. • The entropy change for an isothermal pressure change followed by an isobaric temperature change will be the same as a simultaneous change of both. • (Caveat: we must use the appropriate values of constants or functions (e.g., α at the relevant T; CP at the relevant P). Hess’s Law • Once we have experimentally measured enthalpies of formation for substances, we can calculate enthalpies of reaction using Hess’s Law: ∆ H r = ån i H f ,i i • Where ν is the stoichiometric coefficient (by convention negative for reactants, positive for products) and the sum is over all compounds in the reaction. • So, for the reaction 2MgO + SiO2 = Mg2SiO4 ∆Hr = Hf,Mg2SiO4 - 2Hf,MgO- Hf,SiO2 Standard State Enthalpies • We define the enthalpies of elements (or elemental compounds such as O2) in the standard state of 298.16K and 0.1 MPa (25˚C, 1 atm) as 0. o Units of enthalpies are what? • We can then determine standard states of formation (from the elements) of compounds such as SiO2 as the enthalpy of reaction to form the compounds under standard state conditions: • ∆Hof,SiO2 = ∆Hr where the reaction is: Si + O2 = SiO2