12-Example From Research
advertisement

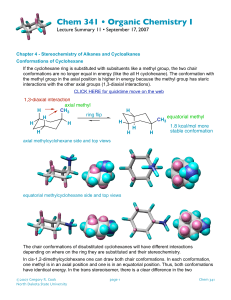
Chemical Exchange Dynamic processes can be observed by NMR if the spectral parameters are changed by the process on the NMR time scale. - Hindered rotation about a partial double bond. - Ring inversion. - Reorientation in solids - Carbonyl scrambling in metal complexes Example - Dimethylacetamide Equally populated 2-site mutual exchange A A CH3 2 O k N N H3C CH3 1 O CH3 1 B H3C CH3 B 2 Slow Exchange k is much smaller than (A - B). Two separate lines at A and B. k = 0 . 1 k = 0 A B Spectrum not sensitive to rate. Rate are measured using T1 based techniques. Intermediate Exchange k of the same order of magnitude as (A - B) The two lines broaden, move towards each other and eventually coalesce 9 9 9 9 . 0 = k 5 . 0 = k A B Spectrum very sensitive to rate Rate measurements are made by spectral simulation Fast Exchange k is larger than (A - B) k = 3 k = 1 . 3 k = 1 . 0 2 A Spectrum becomes insensitive to rate. Rate measurements based on T2 methods Selective Inversion Experiment x RD C B A z VD Acq E D F z A z C B y x y x z y x z D z E y x x x F y x y x Signal of both magnetisation as a function of delay time 1 0 NormalizedSignalItensiy N o n s e l e c t i v e i n v e r s i o n r e c o v e r y I n v e r t e d L i n e U n p e r t u r b e d L i n e 1 0 51 01 52 02 53 03 5 T i m e ( s ) Rate =0.5 s-1 T1= 4 s Accurate rates are possible for 1/T1 < k. Offset-Saturation Experiment Makes it possible to measure accurate T2 values independent of coherent dephasing mechanisms. Decoupler 5xT1 90o Transmitter Acq. 6 0 4 0 1 / 2 = B ( T / T ) 1 / 2 2 1 2 1 / 2 SignalItensiy 2 0 0 6 0 0 0 6 5 0 0 7 0 0 0 7 5 0 0 F r e q u e n c y ( H z ) Accurate Activation Parameters Eyring equation æ- D G † ö kbT ÷ k = exp çç ÷ ÷ çè R T ø h I.e. k ln T ( ) ækb ÷ ö DS † - DH † = + ln çç ÷ + èh ÷ ø RT R k 1 † as a function of one obtains D H from T T † the slope and D S from the y-intercept. Plotting ln ( ) Small temperature range: Large error in slope and hence in y-intercept. Accurate rate measurements are possible in all three exchange regimes: rate data is available over a large temperature range (c.a. 150 oC) D H † to within 1 kJ/mol. D S † to within 5 J/mol K. Eyring Plot of Furfural in Acetone-d6 1 0 b/h) 0 O f f s e t s a t u r a t i o n Ln(k/T)-Ln(k 1 0 L i n e s h a p e 2 0 3 0 0 S e l e c t i v e i n v e r s i o n 1 2 3 4 5 1 1 0 0 0 / T ( K ) 6 7 Large Systems Complications: Spectral overlap Second Order effects Complicated exchange mechanism Both full spectral assignment and the exchange mechanism are required for complete lineshape analysis Solution: Obtain assignment via two-dimensional NMR methods such as: COSY - Correlation spectroscopy NOESY - Nuclear Overhauser Effect Spectroscopy HMBC - Heteronuclear Multiple Bond Correlation Spectroscopy Measure spectral parameters simulation of the complete spectrum simulation of a series of subspectra obtained by selective TOCSY (Total Correlation Spectroscopy) Exchange mechanism determined by Exchange Spectroscopy DADS N,N’-[Dimethyl-(2,2’-dithiobisacetyl)]ethylenediamine CH3 N S S OO N CH3 A challenge Ten-membered ring with 5 observable conformations. 2 Symmetric conformations each with 2 equivalent AB’s and CH3’s and an AA’BB’ 3 Non-symmetric conformations each with 2 non-equivalent AB’s and CH3’s and an ABCD Exchange mechanism of DADS O O MS E (3.1) N 74.1 S N M O M S N O S S N S N O B (0.9) 81.2 M M O N A (3.6) M 71.6 O O M S N S S O D (5.4) S N M 80.1 O N M N M C (0.0) Selective Inversion of the Methyl resonance of Conformer C 3 2 signal(rb.units) 1 0 1 A N m e t h y l C N m e t h y l E N m e t h y l 2 01234567891 0 t i m e ( s ) Data indicates no direct exchange between C and A. Rates were measured between 273 to 283 K Eyring Plot for all Observed Processes of DADS b/h) 2 0 log(k/T)-log(k 2 5 3 0 2 A t o C A t o E A t o B C t o D 3 1 1 0 0 0 / T ( K ) 4 The activation parameters of all four exchange processes of DADS. H† S† (kJ mol-1) (J K-1) G†300 (kJ mol-1) 71.6 +/- 1 3 +/- 3 71.6 C to D 85.7 +/- 1.5 19 +/- 4 80.0 A to B 84.4 +/- 1.2 22 +/- 4 77.8 Process C to A A to E 73.8 +/- 1 11 +/- 3 70.5 Conclusions Barriers were determined for all four processes with errors in the activation parameters compatible to those in studies on small molecules. 9 points on the potential energy surface were determined 2-D methods were invaluable in the application of complete lineshape methods.