Quantum Theory of Solids
advertisement

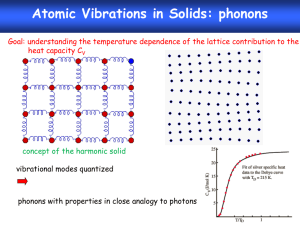
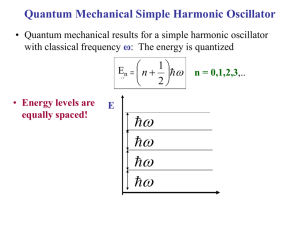
SS206: 5th Lecture Quantum Theory of Solids Classical approach to specific solids predicts that CV is constant at 3R (equi-partition principle). This is known as Dulong –Petit’s Law. This law works very well at high temperature region. But in early twentieth century, low temperature measurements revealed an interestingly different story. experimental temperature dependence of CV for solids We will now discuss about two successful theories which have resolved the issue. Monatomic Crystals Let us consider a one dimensional crystal where atoms are connected by spring. This description works for crystals because each atom/molecule at corresponding lattice site is confined by a step potential. Schematic representation of one dimensional crystal system of masses and springs. In the course of vibration, atoms can displace by small amount, ξj. We can write the energy U of such a crystal of N atoms as a Taylor series expansion in ξj N U U 1 , 2 ,....., n U 0,0,......,0 j 1 j j 0 1 N N 2U 2 i 1 j 1 i j i j ......... 0 The 2nd term on the RHS is zero because U is minimum at zero displacement (equilibrium state). We can write the expansion as U 1 , 2 ,....., n U 0,0,......,0 1 N N Kiji j 2 i 1 j 1 Where we have truncated the series after the quadratic term. Here Kij is the force constant of the bond between the bond involving atom i and j. Also the all the Kij = K, as the crystal is monatomic. Now, this quadratic function can be diagonalized by introducing normal coordinate, just as we do in vibrational spectroscopy of polyatomic molecules. If we have N atoms in the crystals, then 3N-6 vibrational modes will be there. Since here N is of O(1023), then 3N-6 ≈ 3N. So, we will have 3N normal modes of frequency of the jth mode be j 1 2 kj j We do not really need the precise form of kj, μj, and νj in this treatment (we can get them from considering lattice dynamics). We shall replace νj by a distribution which is called phonon density of state (the normal modes are called phonons). Now the crystal does not translate and rotate, so the partition function of the crystals is 3N QN V , T e U0 qvib, j j 1 Here qvib,j is the vibrational partition function (PF) of the jth normal mode. Let us now evaluate the vibrational PF. Consider a harmonic oscillator of frequency ν. Energy levels are 1 E j j h , 2 j = 0,1,2,3………….. Partition function is qvib e Ej j 0 e 1 h 2 e j h j 0 1 h 2 e 1 e h So, the total canonical PF is 12 h j e QN V , T e U0 h j j 1 1 e 3N Also we can write the logarithm of the PF as ln QN V , T h U0 3N h / k ln 1 e j BT j kBT j 1 kBT Now, we introduce a phonon density of states g(ν) which should follow the following equation d g 3N 0 So the logarithm of the PF can be written as the following integral equation ln QN V , T U0 ln 1 e h kBT 0 / kBT 2hk T g d B Now we will try to get integral equation of different thermodynamics properties of the crystal using this PF. Let us get the integral equation for energy of the system E. Using the relation of ln QN V , T canonical ensemble E k BT 2 , we get T V h e h / kB T h g d E U0 h / k B T 2 1 e 0 We can also get an expression for heat capacity CV using the thermodynamics relation E ( CV ) as T V CV k B 0 h / kBT 2 e h / kBT g d 1 e h / k B T 2 At this point given a suitable expression for phonon density of states g(ν), one can get the thermodynamics properties and their temperature dependence. Einstein’s Theory In order to explain non-classical, low temperature behaviour of specific of solids, Einstein proposed a simple quantum model. The idea was that, for a particular crystal all the normal modes have a particular characteristic frequency, νE, termed as Einstein frequency. So, according to the proposal the phonon density of states will take the form g 3N E So the CV will take the form 2 h e h E / kBT CV 3NkB E 2 k BT 1 e h E / kBT Now we will define a characteristic temperature called Einstein temperature (ΘE) for crystal as E h E kB Now the expression of CV will read as a function ΘE e / T CV 3Nk B E 2 T 1 e / T 2 Let us now examine the above equation at two different conditions: 1. At very high temperature regime E 0, T e So, E / T 1 and 1 e E / T 2 1 1 E / T ....... E T 2 CV T 3NkB (This is the dulong-petit’s law of heat capacity, classical limit) 2. At very low temperature regime E T and 1 e E / T 2 1 So, the expression for heat capacity at low temperature regime will be 2 CV T 0 3Nk B E e E / T T 2 Law of corresponding state Before going into the limitation of the theory, we would like to point out one important feature of the above equation. The equation predicts that CV is the same function for all the substances if it is plotted against the reduced temperature T/ΘE. When this happens, we can say that CV is a universal function of T/ΘE, and that the various crystals obey a law of corresponding states. Once the temperature is scaled or “reduced” by a quantity that depends upon the particular substance, the heat capacity versus reduced temperature plots will superimpose for all the crystals. Although the Einstein theory does not quantitatively reproduce the experimental heat capacity data (at low temp regime, we will discuss it next), its prediction of a law of corresponding state is, in fact, correct. The specific heat capacity of various solids as a function of T/ΘE (Adapted from Elements of Statistical Thermodynamics, 2nd edition, by L.K. Nash, Addison-Wesley, 1972.) Limitation of Einstein Theory The temperature dependence of heat capacity derived from Einstein model is capable of giving an impressive qualitative agreement with experiment. However, quantitative agreement is somewhat poor, especially in the low temperature regime. The experimental temperature dependence of the heat capacity follows a T3 law at lower temperature regime. The low temperature dependence of the heat capacity predicted by the theory falls to zero value more rapidly than T3 law. The specific heat capacity of lead showing the disagreement with Einstein's theory at low temperatures. (Adapted from Elements of Statistical Thermodynamics, 2nd edition, by L.K. Nash, Addison-Wesley, 1972.) The reason for this breakdown at low T, low frequency or long wavelength modes get more populated and that needs to be treated explicitly. Debye theory which we will discuss next has solved the issue. Debye Theory of Solids The disagreement between Einstein’s result and the experimental data is due to the fact that Einstein’s assumptions about the atoms in a crystal do not strictly apply to real crystals. The main problem lies in the assumption that a single frequency of vibration characterizes all 3N oscillators. Debye improved on Einstein’s theory by considering the vibrations of a body as a whole, regarding it as a continuous elastic solid. He associated the internal energy of the solid with stationary elastic sound waves. Each independent mode of vibration (or normal mode) is treated as a degree of freedom. In Debye’s theory a solid is viewed as a phonon gas. Vibrational waves are matter waves, each with its own de Broglie wavelength and associated particle. The particle is called a phonon, with characteristics similar to those of a photon. We are interested in determining the number of possible wavelengths or frequencies within a given range. For quantum waves in a one-dimensional box we saw that the wave function is A sin kx , where k 2 n , L n=1,2,3……. Here λ is the de Broglie wavelength, n is the quantum number and L is the dimension of the box. Using the fundamental equation of wave motion, , where is the wave velocity and ν is the frequency, we obtain n 2L 2L If we consider an elastic solid as a cube of volume V = L3 we get n 2V 1/ 3 where, in this case n2 = nx2 + ny2 + nz2 . The quantum numbers nx ,ny and nz are positive integers. Thus the possible values that they can assume occupy the first octant of a sphere of radius n nx2 ny 2 nz 2 1/ 2 A shell of thickness dn of an octant of a sphere of radius n. Let g(ν)dν be the number of possible frequencies in the range ν to ν+ dν . Since n is proportional to ν, g(ν )dν is the number of positive sets of integers in the interval n to n + dn that is, within a shell of thickness dn of an octant of a sphere with radius n: 1 1 g d 4 n 2 dn n 2 dn 8 2 4 V 3 2 d In a vibrating solid, there are three types of waves: one longitudinal with velocity l and two transverse with velocity t . All are propagated in the same direction. When all three waves are taken into account, the above equation becomes 1 2 g d 4V 3 3 2d l t Since each oscillator of the assembly vibrates with its own frequency, and we are considering an assembly of 3N linear oscillators, there must be an upper limit to the frequency spectrum. The maximum frequency νD is determined from the fact that there are only 3N phonons: 3N D 0 1 2 g d 4V 3 3 D3 l t Combining this result with the second last equation, we get g d 9 2 d D3 Now, we can understand the principle difference between Einstein and Debye model is the assumption about the frequency spectrum of the lattice vibration (shown below). Frequency spectra of crystal vibrations: (a) Einstein model; (b) Debye model Now put the expression for g(ν) in the integral expression of CV and we will get CV 9 Nh D3 D 0 h eh / kBT 3d 2 2 h / k T kBT e B 1 Now, we will define x = hν/kBT and Debye characteristic temperature ΘD = hνD/kB. The above equation will take a form after certain step of mathematical manipulation T CV 9 NkB D 3 /T D 0 x 4e x e x 1 2 dx Let us examine the above equation in two conditions 1. At very high temperature regime x 0 , e x 1 x , and e x 1 So, the heat capacity will be T CV 9 Nk B D 3 /T D 0 3 T D x dx 3Nk B D T 3 2 3Nk B This is heat capacity predicted by Dulong-petit’s law (classical equi-partition theory) 2. At very low temperature regime D / T so, the integral equation for CV can be written as T CV 9 Nk B D 3 0 x 4e x e x 1 2 dx The integral appears on the RHS is a standard and equals to 4 4 / 15 . So the expression for CV becomes T 12 4 CV Nk B 5 D aT 3 3 Here a is constant for a particular solid. This is the famous T3-law. This theory predicts the temperature dependence of the heat capacity of monatomic solids in the whole temperature range quiet well both qualitatively and quantitatively. One table will be enough to show the comparison between Einstein and Debye models prediction. Heat capacity of silver at different temperature Recent work has centered on the behavior of solids at low temperatures. Experiments suggest that amorphous materials do not follow the Debye T3 law at temperatures below Debye temperature where quantum effect become important. There is more yet to be learned.