Class 13_BB
advertisement

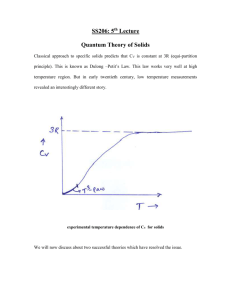
October 21th, 2010 Class 13 Phonon Heat Capacity The heat capacity of a material is the ratio between the heat (energy) provided to the material and the change in its temperature. In other words, the amount of heat necessary to increase the temperature of said material in 1 degree. A good analogy is a liquid container, the “heat” is the water poured into the container, the temperature is the liquid level on the container, the heat capacity is a relationship between the two, how much water you need to add so the level raisee 1 unit. A good analogy for a large heat capacity material in comparison to a small heat capacity is a wide and a narrow container, in a narrow container (small heat capacity), a small amount of water will be enough to raise the level significantly, while for a wide container (large heat capacity), more water is needed to produce the same water level. When heat is added to a material, the energy can be used for many things, one is to excite phonon modes and that is what we will be concerned about here. The heat capacity can be defined at a constant volume or a constant pressure, while experimentally heat capacity is often measured in a constant pressure condition, the heat capacity at a constant volume is more fundamental (since it only involves changes in internal energy as opposed to the case at constant pressure where some of the energy is used to change the lattice volume involving mechanical work). The two heat capacities CV and Cp are related such that Cp-CV=f(T) thus knowing one, the other can be obtained. U CV Where U is the energy. The different contributions to the heat capacity can be T V obtained by taking the derivative with respect to temperature of the corresponding energy. Particularly, the contribution to the heat capacity of the phonon modes, is known as the lattice heat capacity Clat. The total phonon energy (ignoring the zero point energy) is: U lat nK ,p K ,p K p <nK,p> is the average occupancy of each of the phonon modes at thermal equilibrium, K is the wave number related with through the dispersion relation, and p in the polarization (acoustical, optical, transversal, longitudinal) The average occupancy of a given mode is given by the Plank distribution. (See book for the steps leading to this equation) n 1 1 exp k BT Combining the two 1 U lat K p K ,p K ,p 1 exp k BT At this point it is important to remember that the K’s are discrete but too close to each other and can be considered a continuum, but they form a continuum and so an integral is more appropriate than a summation. If the number of modes in the frequency range +d with polarization p is given by Dp()d, then U lat dD p p 1 exp k BT Where of course the dispersion relation allows us to integrate in instead of K. Thus Clat Where x U lat x 2e x k B dD p T p e x 12 k BT Now the heat capacity can be obtained if the density of modes is known. Coming up next, we revise some models to determine the density of states and with that to solve the integral and obtain the density of states. Density of States in One Dimension The values of K that are possible in a given lattice would be a continuum unless appropriate boundary conditions are applied. One common type is the periodic boundary condition, in one dimension that simply means that the first and last point in the chain are entirely equivalent (thus starting the sequence again). A condition like that implies u(sa)= u(sa+L) By imposing this condition to the phonon wave function in a line of N+1 sites, such that site 1 and site N+1 are equivalent by the boundary conditions (N independent sites) uoei(Ksa-(K)t)= uoei(K(sa+L)-(K)t)=uoei(Ksa-(K)t) eiKL What implies that eiKL=1 or KL=2n or 2 2 4 6 N , , , , Notice that for ±N/L (where L/N=a) we have e±i where + L L L L and - are actually the same angle and thus the same solution, so there are exactly N independent solutions. For n > N the same solutions are obtained. K 0, K 0, 2 4 6 N , , ,, L L L L Thus for a chain of N+1 atoms with periodic boundary conditions, there are exactly N states, 1 per each atom moving independently in the chain. Also notice that K=±n/L actually refers to a wave with the same frequency only propagating in opposite directions. The distance between two points in the K-space is 2/L. The number of states per unit of length in the K space is then L/2 and thus the number of states of absolute value in the range [0,K] is then given by NK 2 L K 2 (where the 2 account for the two waves with the same K propagating in opposite direction ±n/a). The density of states, “number of states per unit of length” can be defined as dN/dK for N large enough that the states can be consider a continuum. So D d dN K dN K dK L dK L d d 2 d dK d 2 d d dK The dependence of the density of states in terms of ω has been forced, but now the density of states is expressed in terms of the group velocity which can be obtained if the dispersion relation is known. Density of States in Three Dimensions By applying periodic boundary conditions in the same way that we did for 1-D, the number of states inside a sphere of radius K is: 3 3 L 4K where L3=V N K 2 3 Thus the density of states is then given by: D d dN K dN K dK VK 2 d d d d dK d 2 2 d dK 3 The problem is now reduced to finding the group velocity (or the dispersion relation) for a particular crystal and to apply the equations above. Debye Model for the Density of States In this model, the velocity of sound is taken as a constant for each polarization type, thus VK 2 1 V 2 p K and D 2 2 d 23p dK Now we have all the ingredients and we can calculate the lattice energy (under the Debye approximation). V 2 d d U lat dD p d 2 3 2 kBT 0 0 p p e e kBT 1 p 1 Where D is the Debye frequency and correspond to that frequency associated with the maximum value of K, KD. Now, if we assume that the velocity is independent of the polarization mode and we consider three polarization modes (3 acoustical, 1 per dimension) 3Vk 4T 4 xd 3 x3 3V d U lat 2 3 d 2 B 3 3 dx x 0 e 1 2 0 e k BT 1 2 Where the following variable substitutions has been made from where Here it is convenient to introduce another definition, the Debye temperature what leads to The Debye temperature is then the temperature at which xD=1 or the temperature at which the maximum phonon energy is equal to kBT. 3 3 L 4 Now using that N K to obtain an expression for . 3 2 3 Where N is the total number of occupied states With these definitions, now U lat 3 3V d T 2 3 d 9 Nk BT 2 0 e kBT 1 3 xd 0 4 x3 T d x Nk BT e 1 15 What leads to 4 3 CV U lat 233.783 3 T T 3 This is known as the Debye T3 approximation. See figure 9, page 115 for the heat capacity of Argon, where this law is in very good agreement with the experiment. For Argon, the Debye temperature is 92K. The agreement with this law worsens as T approaches the Debye temperature, the law is most approximately met for T within 1-2% of the Debye temperature. Thus the Debye approximation is good for very low temperature (compare to the Debye temperature). Einstein Model of the Density of States This model considers N oscillators all vibrating at the same frequency o and thus D()=Nδ(-o) U lat dN o 3N o o p e kBT 1 e kBT 1 o CV 3NkB 2 o 2 k BT o k BT e 1 For large T, CV~3NkB, the Dulong and Petit Value. In summary, the Einstein model accounts for the high temperature limit while the Debye model accounts for the low temperature regime. 5