The Heat Capacity of a Solid 6.1 INTRODUCTION 6.2 EINSTEIN'S
advertisement

The Heat Capacity of a Solid
6.1 INTRODUCTION
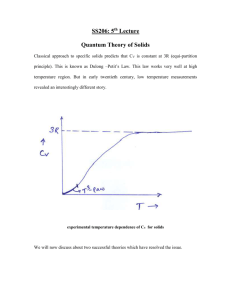
The investigation of the heat capacity of solids is important in the study of condensed matter.
This example, like the diatomic case, illustrates the shortcomings of classical kinetic theory
and the need for statistical mechanics and quantum theory to provide answers that agree with
experimental results. In 1819, Dulong and Petit observed that the specific heat capacity at
constant volume of all elementary solids is approximately 2.49 "10 4 J kilomole#1 K#1 at
temperatures near room temperature. This result can be explained by the principle of
equipartition of energy by treating every atom of the solid as a linear oscillator with six
degrees of freedom and then associating an energy of 12 kT with each degree of freedom. Then
c v = 6( R 2) = 3R , in agreement with the observation of Dulong and Petit.
However, additional measurements showed that the specific heat of solids varies with
temperature, decreasing to zero as the temperature approaches zero. This behavior cannot be
explained by the “freezing” of degrees of freedom when the temperature is decreased since
the specific heat varies gradually with temperature and does not exhibit abrupt jumps by any
multiple of 12 R (in contrast to the specific heat of a diatomic gas). Even at room temperature
the specific heat capacities of certain substances such as beryllium, boron, carbon, and silicon
were found to be much smaller than 3R . Quantum statistics is needed to explain these
discoveries.
6.2 EINSTEIN’S THEORY OF THE HEAT CAPACITY OF A SOLID
It was Einstein who developed the first reasonably satisfactory theory of the specific heat
capacity of a solid. He assumed that the crystal lattice structure of a solid comprising N
atoms can be treated as an assembly of 3N distinguishable one-dimensional oscillators (three
oscillators for each atom since the atoms of a solid are free to move in three dimensions).
From Equation (5.8), the internal energy of a solid made up of N atoms is
1
$1
1 '
U = 3Nk" E & + " E T ) ,
%2 e
#1(
(6.1)
where " E is the Einstein temperature given by " E # h$ k . The heat capacity at constant
volume is
# "U &
# ) & 2 e) E T
CV = % ( = 3Nk% E (
$ "T 'V
$ T ' (e) E T *1) 2
(6.2)
For temperatures very large compared with the Einstein temperature,
CV " 3Nk = 3nR .
(6.3)
Thus the high temperature limit of Einstein’s equation gives the value of Dulong and Petit.
The failure of their law becomes evident when we examine the low temperature limit. For
" E T >> 1,
# " &2
CV = 3Nk% E ( e)" E
$T'
T
(6.4)
As T approaches zero, CV also goes to zero, since the exponential decay overpowers the
growth of (" E T) 2 .
Einstein’s theory also explains the low heat capacities of some elements at moderately high
temperatures. If an element has a large Einstein temperature, the ratio " E T will be large even
for temperatures well above absolute zero, and CV will be small. For such an element
" E # h$ k must be very large and, accordingly, " must be large. For an oscillator with force
constant " and reduced mass µ , the oscillator frequency is
"=
1 $
2# µ
(6.5)
A large frequency value suggests a small reduced mass or a large force constant,
corresponding to lighter elements and elements that produce very hard crystals. The theory
correctly predicts the failure of the law of Dulong and Petit for those elements. As an
example, the heat capacity of diamond approaches 3Nk only at extremely high temperatures
( " E = 1450 K for diamond).
2
The essential behavior of the specific heat capacity of solids is incorporated in the ratio " E T .
When this ratio is large, the partition function reduces to the zero-point term, implying that all
the atoms are in the ground state and the vibrational degrees of freedom are not excited
(Equation (5.6)). The specific heat remains close to zero since small temperature increases are
not sufficient to excite a significant number of atoms to the first vibrational state. When " E T
is small, the difference between energies corresponding to various vibrational states is small
compared with thermal energies. Thus the vibrational states can be approximated by an
energy continuum and treated by classical theory. For values of " E T between the two
extremes, there is a transition region of partial excitations.
A consequence of the fact that
CV N depends only on the
ratio " E T is that a single
measurement of the heat
capacity at one temperature
determines its value at all
other temperatures. In
Figure 6.1 The specific heat capacity of various solids as a function of
addition, different elements at
" E T . Note that c v = (N A N )CV . (Adapted from Elements of
different temperatures will
Statistical Thermodynamics, 2nd edition, by L.K. Nash, AddisonWesley, 1972.)
possess the same specific heat
capacity if the ratio " E T is
the same in each case. The elements are said to be in “corresponding states”. The graph in
Figure 6.1 clearly shows this behavior.
Careful measurements of heat
capacities show that Einstein’s model
gives results that fall slightly below
experimental values in the transition
range of " E T between the two
limiting values. The discrepancy can
be seen in Figure 6.2 in which the heat
capacity of lead is shown for
temperatures in the range 0 " 50 K .
Figure 6.2 The specific heat capacity of lead showing the
disagreement with Einstein's theory at low temperatures.
(Adapted from Elements of Statistical Thermodynamics, 2nd
edition, by L.K. Nash, Addison-Wesley, 1972.)
3
6.3 DEBYE’S THEORY OF THE HEAT CAPACITY OF A SOLID
The disagreement between Einstein’s result and the experimental data is due to the fact that
Einstein’s assumptions about the atoms in a crystal do not strictly apply to real crystals. The
main problem lies in the assumption that a single frequency of vibration characterizes all 3N
oscillators. Debye improved on Einstein’s theory by considering the vibrations of a body as a
whole, regarding it as a continuous elastic solid. He associated the internal energy of the
solid with stationary elastic sound waves. Each independent mode of vibration (or normal
mode) is treated as a degree of freedom.
In Debye’s theory a solid is viewed as a phonon gas. Vibrational waves are matter waves,
each with its own de Broglie wavelength and associated particle. The particle is called a
phonon, with characteristics similar to those of a photon. We are interested in determining the
number of possible wavelengths or frequencies within a given range.
For quantum waves in a one-dimensional box we saw that the wave function is " = Asin kx ,
where
k=
2" n"
=
, n = 1,2,3,...,
#
L
(6.6)
Here " is the de Broglie wavelength, n is the quantum number and L is the dimension of the
box. Using the fundamental equation of wave motion, c = "# , where c is the wave velocity
and " the frequency, we obtain
n=
2L 2L
=
#
"
c
If we consider an elastic solid as a cube of volume V = L3 we get
n=
2V 1 3
"
c
(6.7)
where, in this case n 2 = n x2 + n y2 + n z2 . The quantum numbers n x , n y and n z are positive
integers. Thus the possible values that they can assume occupy the first octant of a sphere of
radius
4
12
n = ( n x2 + n y2 + n z2 )
Let g(" )d" be the number of possible frequencies
in the range " to " + d" . Since n is proportional
to " , g(" )d" is the number of positive sets of
integers in the interval n to n + dn that is, within
a shell of thickness dn of an octant of a sphere
with radius n :
g(" )d" =
1
1 2
2
4#n
{ dn
{ = #n dn .
8 Surface thickness 2
{
octant area
of shell
Figure 6.3 A shell of thickness dn of an octant
of a sphere of radius n .
Substituting Equation (6.7) for n , we obtain
g(" )d" =
4#V 2
" d" .
c3
(6.8)
In a vibrating solid, there are three types of waves: one longitudinal with velocity c l and two
transverse with velocity c t . All are propagated in the same direction. When all three waves
are taken into account, Equation (6.8) becomes
$1 2'
g(" )d" = 4#V & 3 + 3 )" 2 d" .
%c l c t (
(6.9)
Since each oscillator of the assembly vibrates with its own frequency, and we are considering
an assembly of 3N linear oscillators, there must be an upper limit to the frequency spectrum.
The maximum frequency " m is determined from the fact that there are only 3N phonons:
3N =
#
"m
0
g(" )d" =
4$V % 1
2( 3
' 3 + 3 *" m
3 &c l c t )
(6.10)
Combining this result with Equation (6.9), we get
9N" 2 d"
g(" )d" =
" m3
(6.11)
5
Equation (6.10) provides us with some insight into the cutoff frequency and wavelength.
Since " m # (N V )1 3 and "min #1 $ m , it follows that
$ V '1 3
"min # & )
%N(
The minimum possible wavelength is determined by the average interatomic spacing. Thus
the structure of the crystal sets a lower limit to the wavelength; shorter wavelengths do not
lead to new modes of atomic vibration.
The principal difference between Einstein’s description and Debye’s model is in the
assumption about the frequency spectrum of the lattice vibrations. This is shown graphically
in Figure 6.4. More rigorous calculations have led to more complex spectra.
Now, there is no restriction on the number of phonons per energy level jh" , where j is an
integer. Thus phonons are bosons.1 This means that the occupation numbers must be given by
the Bose-Einstein distribution. For the continuum, Equation (2.41) applies:
1
N(")
= (" # µ ) kT
#1
g(") e
In this expression the chemical
potential µ must be set equal
to zero. This is because the
total number N of phonons is
not an independent variable
but rather is determined by the
volume and temperature of the
particular crystal being
Figure 6.4 Frequency spectra of crystal vibrations: (a) Einstein
considered. Specifically, N is
model; (b) Debye model.
the number of phonons that
causes the Helmholtz function to be a minimum at equilibrium. Since µ = ("F "N )T ,V , it
follows that µ = 0 . With " = h# , we have
N(" )d" =
1
g(" )d"
e h" kT #1
(6.12)
Some physicists like to call them “phony bosons”.
6
where N(" )d" is the number of phonons with frequencies in the range " to " + d" . When
we substitute Equation (6.11) in this result, we obtain
% 9N " 2 d"
'
, " $ "m
N(" )d" = & " m3 e h" kT #1
'(
0,
" > "m
(6.13)
The total energy of the phonons in the frequency range " to " + d" is h" N(" ) d" . Hence the
internal energy of the assembly is
U=
#
"m
0
h" N(" )d" =
9Nh
" m3
#
"m
0
" 3 d"
e h" kT $1
(6.14)
(We leave out the constant zero-point energy since this term has no effect on the heat
capacity. Its value is calculated in Problem 6.2.)
The Debye temperature " D is defined as
"D #
h$ m
k
(6.15)
That is, the Debye temperature is proportional to the cutoff frequency " m . Some values are
given in Table 6.1
TABLE 6.1 Debye temperatures
of some materials.
"D
Substance
(K)
Lead
88
Mercury
97
Sodium
172
Silver
215
Copper
315
Iron
453
Diamond
1860
7
To obtain the heat capacity, we need to differentiate Equation (6.14) with respect to the
temperature. Now
' h"
1
e h" kT
d $
)=
&
dT % e h" kT #1( kT 2 (e h" kT #1) 2
Thus
dU 9Nh
= 3
CV =
"m
dT
$
"m
0
hv
e h" kT
3
2 v dv
2
h
"
kT
kT (e
#1)
(6.16)
We let x = h" kT and x m = h" m kT = # D T . With the change of variable we have
dx = (h kT)dv so
9Nh 2 1
CV = 3
" m kT 2
$
e h"
"m
0
(e
h" kT
kT
9Nh 2 1
2 v dv =
" m3 kT 2
#1)
4
5
9Nkh 2 1 % kT ( + D
=
' * $
" m3 (kT) 2 & h ) 0
# T &3 " D
CV = 9Nk% ( * 0
$ "D '
T
T
x 4 e x dx
(e x )1)
2
$
+D T
0
% kTx ( 4 % kT (
* ' * dx
2'
(e x #1) & h ) & h )
ex
% kT ( 3 + D
* $
2 x dx = 9Nk'
& hv m ) 0
(e x #1)
ex
.
4
T
e x x 4 dx
(e
x
#1)
2
(6.17)
For high temperatures, " D T << 1 and x << 1 . So e x "1 # x in the denominator and e x " 1 in
the numerator, so the integral becomes
#
"D T
0
3
1 $ "D '
x dx = & )
3% T (
2
Hence
# T &3 1 # "D &3
CV = 9Nk% ( % ( = 3Nk
$ "D ' 3 $ T '
This is the law of Dulong and Petit. For low temperatures, " D T is large and we can let the
upper limit of the integral be infinity. Then
8
$
#
0
x 4 e x dx
4% 4
2 =
(e x "1) 15
(6.18)
and
!
12" 4 $ T '
CV =
Nk& )
5
% #D (
3
(6.19)
This equation is known as Debye’s T 3 law. It is valid when the temperature is lower than
!
about 0.1" D , which means for most substances about 10-20 K. The relation gives a better fit
to experimental data at very low temperatures than the Einstein model, and is valid for all
! temperature is above the Debye temperature, the heat capacity is
monatomic solids. When the
!
very nearly equal to the classical value 3Nk .
For temperatures below the Debye
temperature, quantum effects become
! zero. Note that
important and C decreases to
V
diamond, with a Debye temperature of 1860
K, is a “quantum solid” at room temperature.
!
Figure The frequency spectrum for Tungsten
Recent work has centered on the behavior of
compared with the Debye theory with " D = 367 K
solids at low temperatures. Experiments
suggest that amorphous materials do not
3
follow the Debye T law !
even at temperatures below 0.01" D . There is more yet to be learned.
!
!
9