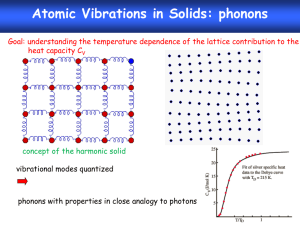
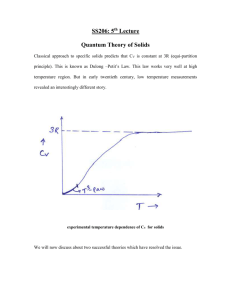
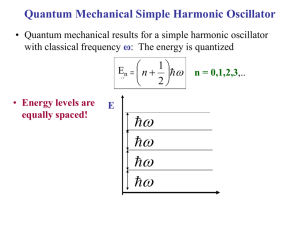
Heat Capacity with Phonons
advertisement
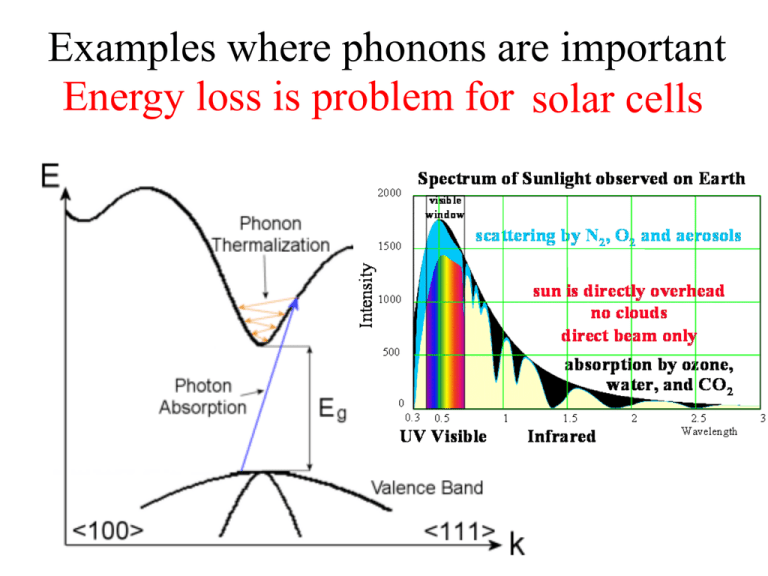
Examples where phonons are important Energy loss is problem for solar cells Indirect semiconductors Describe differences. Low energy transition can only be excited if phonons present Real Phonon Spectra Might Look Slightly Different Why? We made several simplifications: •Interactions beyond nearest neighbors are not included •Assumed harmonic potential •Ignored electron-phonon coupling Predict for Diamond along one direction (try 001) http://exciting-code.org/phonon-properties-in-diamond-structure-crystals Longitudinal Optical Transversal Optical degenerate Longitudinal Acoustic (0,0,0) 1 1 1 ( , , ) 4 4 4 fcc lattice with basis Transversal Acoustic degenerate P=2 2x3=6 branches expected Can you guess what this is? What makes this look quite different? How could we measure this? All measurements before from neutrons. Neutron Diffraction for Phonons Neutrons were discovered in 1932 and their wave properties was shown in 1936. E = p2/2m p = h/λ E=Energy λ=Wavelength p=Momentum mn=Mass of neutron = 1.67x10-27kg λ ~1A° Energy E~0.08 eV. This energy is of the same order of magnitude as the thermal energy kT at room temperature, 0.025 eV, and for this reason they are good at probing phonons. Diffraction Methods Diffraction X-ray Neutron Electron The physical basis for the diffraction of electron and neutron beams is the same as that for the diffraction of Xrays, the only difference is the mechanism of scattering. Neutron Diffraction • Neutron has no electrical charge. Interaction is with atomic nuclei (strong force, short range) • Neutron-matter interaction is weak (mean free path ~1 cm) • Although uncharged, neutron has an intrinsic magnetic moment, so it will interact strongly with atoms and ions in the crystal which also have magnetic moments. • Neutrons are more useful than X-rays for determining the crystal structures of solids containing light (low atomic number) elements. • Neutron sources in the world are limited so neutron diffraction is a very special tool. Oak Ridge, Tennessee Start with negatively charged hydrogen ions (1 proton and 2 electrons), produced by an ion source. Ions injected into a linear accelerator. The ions pass through a foil, which strips off the electrons, converting it to a proton. The high-energy protons strike a target of liquid mercury, where spallation occurs. The spalled neutrons are guided through beam lines. Very expensive and involved experiments (construction of Oak Ridge neutron source was ~ $1 billion) Table top alternatives ? Yes, infrared absorption and inelastic light scattering (Raman and Brillouin) However only q~0 accessible (atomic scattering factors decrease as q2 for light). See ch. 7 of http://www.slideshare.net/dasepbux/wavediffraction-53527785 Lonely scientist in the reactor hall Phonon spectroscopy = Conditions for: in elastic scattering Constraints: Conservation laws of Momentum Energy In all interactions involving phonons, energy must be conserved and crystal momentum must be conserved to within a reciprocal lattice vector. Magnetic Applications of Neutron Diffraction Establishing magnetic structure of materials – Neutrons carry magnetic moments and their scattering is affected by magnetic moments of atoms Example: Mn Magnetism of MnO Mn2+ ions face-centered cube O2- ions in the centers of all edges and in the body center What crystal structure? What happens to the unit cell when you add the magnetic structure? O Magnetic Super-Structures (cubic symmetry) c c a Ferromagnetic (FM)=has a spontaneous magnetization whose direction can be changed with a magnetic field Magnetic and charge have the same unit cell a Anti-Ferromagnetic=FM in one plane but antiparallel in next plane, M=0 Magnetic and charge have the different unit cells. Magnetic unit cell doubles nuclear unit cell. Compare the a can c lattice parameters to the non-magnetic version in the case of MnO. Mn cnonmagnetic O anon-magnetic XRD of MnO (from Kittel) What do you expect to happen? Chemical unit cell (FCC) (all odd or even h,k,l) Magnetic unit cell 15 Like Telescope Time, Beamtime is Competitive 3 pages to describe why your work is important, what you expect to learn, how you will do it and why you can’t do it anywhere else! Ranking scheme: 1. Must do 2. High Priority 3. Medium Priority 4. Low priority 5. Don’t do Due ~ 1 year before the work is done! Cutoff If You Do Get Time Magnetism experts XAS expert me I = Installation/Maintanence You might get three days to do all of your experiments for the year! Heat Capacity Revisited (now with phonons) Revisiting Heat Capacity • Dustin will discuss a summary of heat capacity next time, so let me skip to the focus of today • Drude (spring-like) -> constant heat capacity • Sommerfield: linear T dependence At low T quantum phenomena are important and must be taken into account. Specific heat tends to classical value at high temperatures. Lattice Vibrational Contribution to the Heat Capacity The thermal energy is the dominant contribution to the heat capacity in most solids. In non-magnetic insulators, it is the only contribution. Some other contributions: Conduction Electrons in metals & semiconductors. The magnetic ordering in magnetic materials. Thermal Energy & Heat Capacity Quantum Model According to Quantum Mechanics if a particle is constrained; • the energy of particle can only have special discrete energy values. • it cannot increase infinitely from one value to another. • it has to go up in steps. Treating Phonons like QM Oscillator Einstein Model = Heat capacity C can be found by differentiating the QM average phonon energy Pn n The energy of a harmonic oscillator and hence of a lattice mode of angular frequency at temperature T n What happens to as the temperature increases? The probability of the oscillator being in this level as given by the Boltzman factor exp( n / kBT ) Energy of a single oscillator 1 n n 2 Average energy _ Pn n n P n n 1 1 n exp n / k BT _ 2 2 n 0 1 exp n / k BT 2 n 0 1 z exp[( n ) ] 2 k BT n 0 Partition function z e / 2 k BT e 3 z e / 2 k BT z e / 2 k BT What is dz/dT? / 2 k BT e 5 / 2 k BT (1 e / k BT e 2 / k BT (1 e / k BT 1 ) ..... ..... Will need soon According to the Binomial expansion for x«1 where xe h / k BT 1 x 1 x n 0 n 1 1 n exp n / k T B _ 2 2 n 0 1 exp n / k T B 2 n 0 Can be written as: 1 z k BT 2 (ln z ) z T T _ e / 2 k BT 2 k BT ln T 1 e / kBT _ k BT 2 / 2 k BT / k BT ln e ln 1 e T _ / k BT k BT 2 ln 1 e T 2 k T T B k B / k BT 2k k 2T 2 e 1 _ e 2 B B k BT 2 2 / k BT 4 k BT 1 e 2 1 e _ k BT 2 _ Finally, the result is 1 2 e x' (ln x) x x / k BT / k BT / k BT 1 _ 1 2 e / k BT 1 This is the Mean Phonon Energy. The first term in the above equation is the zero-point energy. As mentioned before even at 0ºK atoms vibrate in the crystal. The number of phonons is given by the Bose-Einstein distribution as k BT 1 f ( ) e 1 2 T kBT 1 _ (number of phonons) x (energy of phonon) = (second term in ) The second term in the mean energy is the phonon contribution to the thermal energy. Heat Capacity C (Einstein Model) • Heat capacity C can be found by differentiating the average phonon energy 1 2 e kBT 1 _ d Cv dT Let kB k BT e kBT e 2 1 k 2 kBT Cv k B 2 kBT 2 e kBT e eT Cv k B T e T 1 2 2 k BT 1 2 eT Cv k B T e T 1 2 where 2 k Specific heat in this approximation vanishes exponentially at low T and tends to classical value at high temperatures. Cv kB 2 Area = kB T These features are common to all quantum systems; the energy tends to the zero-point-energy at low T and to the classical value of the Boltzmann constant at high T. The difference between classical and Einstein models comes from zero point energy. Einstein Model Fails at Low Temp • Einstein model also gave correctly a specific heat tending to zero at absolute zero, but the temperature dependence near T= 0 did not agree with experiment. Why? • Taking into account the actual distribution of vibration frequencies in a solid this discrepancy can be accounted for Einstein was aware that getting the frequency of the actual oscillations would be different. He proposed this theory because it was a simple demonstration that QM could solve the specific heat problem. CV of Diamond Finding the 3d density of states D(E) for Photons g (k ) k 2 2 Good analogy for low temp phonons. Why? g (kg(k) )dk gg(E) ( E )dE g(E) dE g(k) g (k ) g ( E ) dk Number of States N=gV (for Photons) How about for Phonons? V2 1 V2 1 2 Ng 3 Ng 2 3 2 3 2 vs 2 vL vT N Factor of 3 from one longitudinal and 2 transverse Debye approximation has two main steps 1. Approx. dispersion relation of any branch by a linear extrapolation 2. Ensure correct number of modes by imposing a cut-off frequency D, above which there are no modes. The cut-off freqency is chosen to make the total number of lattice modes correct. Since there are 3N lattice vibration modes in a crystal having N atoms, we choose D so that: Einstein Debye D approximation to the dispersion approximation to the dispersion vk g ( )d 3N 0 V 1 2 ( )D3 3 N 2 3 3 6 vL vT V 1 2 D 2 ( 3 3 ) d 3N 2 2 vL vT 0 V 1 2 3N 9N ( ) 3 2 2 vL3 vT3 D3 D3 g ( ) 9N D3 2 g ( ) / 2 Accordingly, the Debye spectrum may be written as 9 N 2 3 g ( ) D 0 for for Einstein Debye D D Actual Notes: The Debye spectrum is only an idealization of the actual situation obtaining in a solid; it may be compared with a typical spectrum. While for low-frequency modes (the so called acoustical modes) the Debye approximation is reasonably valid, there are serious discrepancies in the case of high-frequency modes ( the optical modes). For “averaged” quantities, such as the specific heat, the finer details of the spectrum are not very important. Though not as common, the longitudinal and the transverse modes of the solid should have their own cut-off frequencies, D,L and D,T say, rather than D. Accordingly, we should have: D ,L D ,T 2 d 2 d V 2 2cL3 N and 2 2cT3 2 N having a common cut-off at Debye Lattice Energy and Heat Capacity 1 E ( / kBT )g ( )d 2 e 1 0 The lattice vibration energy of becomes and, 9N E 3 D g ( ) 9N D3 2 D D 3 3 1 9 N 2 0 ( 2 e / kBT 1) d D3 0 2 d 0 e / kBT 1d D 9 9N E N D 3 8 D D 3d e / k BT 0 1 First term is the estimate of the zero point energy, and all T dependence is in the second term. The heat capacity is obtained by differentiating above eqn wrt temperature. dE 9 N CD 3 dT D D 0 4 e / k BT d 2 2 kBT e / kBT 1 2 T CD 9 Nk B D 3 /T D 0 4 x e e x x k BT d kT dx kT x define the Debye temperature x 1 2 dx D D kB How does C D limit at high and low temperatures? T >> D High temperature x2 x3 e 1 x 2! 3! T CD 9 Nk B D x x (1 x) x (1 x) ~ 2 x 2 2 2 x x e 1 1 x 1 4 x 4 xe T >> 4 T D CD 9 Nk B D x 3 /T D 0 3 /T D 0 x 4e x e x 1 2 dx is small k BT x 2 dx 3Nk B T << D Low temperature For low temperature the upper limit of the integral is ~infinite; the integral is then a known integral of 4 4 /15 . T << T D CD 9 Nk B D We obtain the Debye T 3 /T D 0 3 x 4e x e x 1 2 dx law in the form 12 Nk B 4 T CD 5 D 3 D D kB How good is the Debye approximation at low T? 1 2 e 0 / kT g d 1 1 d 2 2 k BT 2 Cv V kB 3 3 dT 15 v v L T 3 The lattice heat capacity of solids varies as T 3at low temperatures; Debye law. Excellent agreement for non-magnetic insulators! Motivation for Debye: The exact calculation of DOS is difficult for 3D. Debye obtained a good approximation to the heat capacity by neglecting the dispersion of the acoustic waves, i.e. assuming s k for arbitrary wavenumber. In a 1D crystal, Debye’s approximation gives the correct answer in both the high and low temperature limits. This approximation is still widely used today. Lattice heat capacity due to Debye scheme T CD 9 Nk B D 3 D / T 0 x 4e x e x 1 2 dx C 3 Nk B T Debye formula gives quite a good representation of the heat capacity of most solids, even though the actual phonondensity of states curve may differ appreciably from the Debye assumption. 1 Lattice heat capacity of a solid as predicted by the Debye interpolation scheme 1 T / D Debye frequency and Debye temperature scale with the velocity of sound in the solid. So solids with low densities and large elastic moduli have high D . Debye energy D can be used to estimate the maximum phonon energy in a solid. Solid Ar D ( K ) 93 Na Cs Fe Cu Pb C KCl 158 38 457 343 105 2230 235 Summary: Debye Specific Heat 1 c 3 Nk For T>>D V Same as a classical non-interacting gas 1 4 T 3Nk V 5 Quantum effects important For T<<D cT 4 3 The assumptions of Debye theory are: • the crystal is harmonic • elastic waves in the crystal are non-dispersive • the crystal is isotropic (no directional dependence) • there is a high-frequency cut-off determined by the number of degrees of freedom Comparing Phonon and Electron contributions to heat capacity (for metals) Phonon contribution Electron contribution 1 d 2 2 k BT 2 Cv V kB 3 3 dT 15 v v L T 3 Cv= 𝜋2𝑘𝐵 𝑇 2 𝑇𝐹 T > Debye temp • If each unit cell contributes one or two electrons, then the ratio is about kBT/EF (small for most metals) • Thus, electrons do not contribute much to room temperature heat capacity (significant at low temp) Class Discussion Experimentally, the specific heat of EuO at very low temperatures is proportional to T3/2. • Is EuO a metal or an insulator? Why? • Any idea what type of elementary excitation this behavior results from? (you may not know) • What was the temperature dependence in metals? • Remember what materials Debye was good for? • Magnons with an energy dependence of k2 How good is the Debye approximation at low T? 1 d 2 2 k BT 2 Cv V kB 3 3 dT 15 v v L T The lattice heat capacity of solids thus varies as T 3 at low temperatures; this is 3 referred to as the Debye T law. Figure illustrates the excellent agreement of this prediction with experiment for a non-magnetic insulator. The heat capacity vanishes more slowly than the exponential behaviour of a single harmonic oscillator because the vibration spectrum extends down to zero frequency. 3 Reminder: High and Low Temperature Limits 3NkBT • Each of the 3N lattice modes of a crystal containing N atoms d C dT This result is true only if T >> C 3NkB kB At low T’s only lattice modes having low frequencies can be excited from their ground states; long Low frequency sound waves 0 k a vs k vs k Calculating Specific Heat Capacity 1 2 e 0 / kT 1 2 e 0 g d 1 / kT Zero point energy = z 2 V 1 2 2 3 3 d 1 2 vL vT x V 1 2 3 z 2 3 3 / kT d 2 vL vT 0 e 1 3 V 1 2 k BT 4 z 2 3 3 3 2 vL vT 15 4 e 0 e 3 / kT 0 / kT 3 1 1 d d 0 k BT 3 k BT 3 x kT B dx x e 1 k BT k BT x d k BT dx 4 x3 0 e x 1dx 4 15 1 2 kBT d 2 2 Cv V kB 3 3 dT 15 v v L T 3 at low temperatures Example Calculate the temperature at which the photon energy density in a solid will exceed the phonon energy density in the same solid, if the solid has a Debye temperature of 300K, a phonon velocity of 400,000 cm/s and an index of refraction of n=2 and assuming the solid does not melt. Should we consider the high or low temperature regime? Heat capacity of the free electron gas • From the diagram of n(E,T) the change in the distribution of electrons can be resembled into triangles of height ½ g(EF) and a base of 2kBT so the area gives that ½ g(EF)kBT electrons increased their energy by kBT. The difference in thermal energy from the value at T=0°K E (T ) E (0) ~ 1 g ( EF )(k BT ) 2 2 • Differentiating with respect to T gives the heat capacity at constant volume, E Cv g ( EF )k B 2T T g (E) E E 2 V N g ( E ) dE N EF g ( EF ) 0 0 2 2 3 3 N 3N g ( EF ) 2 EF 2k BTF F F 3N Cv g ( EF )k B T k B 2T 2k B TF 2 V 2 2 3/ 2 1/ 2 (2 m ) E dE 3 3/ 2 1/ 2 (2 m ) E 3 V 3 2 3/ 2 (2 mE ) F 3 T 3 Cv Nk B 2 TF Heat capacity of Free electron gas The energy of diffraction methods Electron X-Ray Neutron λ = 1A° λ = 1A° λ = 2A° E ~ 104 eV E ~ 0.08 eV E ~ 150 eV interact with electron interact with nuclei interact with electron Penetrating Highly Penetrating Less Penetrating ~ Bulk Measurement Bulk Measurement if sample is thin (<100s nm) Surface Sensitive kBT Mean energy of a harmonic oscillator as a function of T 1 2 T Low Temperature Limit kBT 1 2 e kBT 1 _ _ 1 2 Since exponential term gets bigger Zero Point Energy kBT Mean energy of a harmonic oscillator as a function of T 1 2 x2 e 1 x .......... 2! x T High Temperature Limit << kBT is assumed independent of frequency of oscillation by the Einstein method. This is the classical limit because the energy steps are now small compared with the energy of the harmonic oscillator. Notice that is the thermal energy of the classical 1D harmonic oscillator. e 1 k BT _ 1 2 1 1 k BT _ 1 k BT 2 k BT _ kBT When k is not small, DOS bigger Total energy determination tricky Gradient goes to zero at edges of Brillouin Zone Like electronic density of states, there will be a van Hove singularity in the phonon density of states. Another View of the Low Temperature Limit At low T’s only lattice modes having low frequencies can be excited from their ground states, since the Bose-Einstein function falls to zero for small values of Low frequency sound waves at low T 0 vs k vs k k a Vk 2 dk g 2 2 d k 1 dk 1 vs k vs d vs 2 V 2 vs 1 g 2 2 vs vs depends on the direction V2 1 V2 1 2 g g 3 2 3 2 3 2 vs 2 vL vT