Counting Atoms & Balancing Equations Worksheet
advertisement
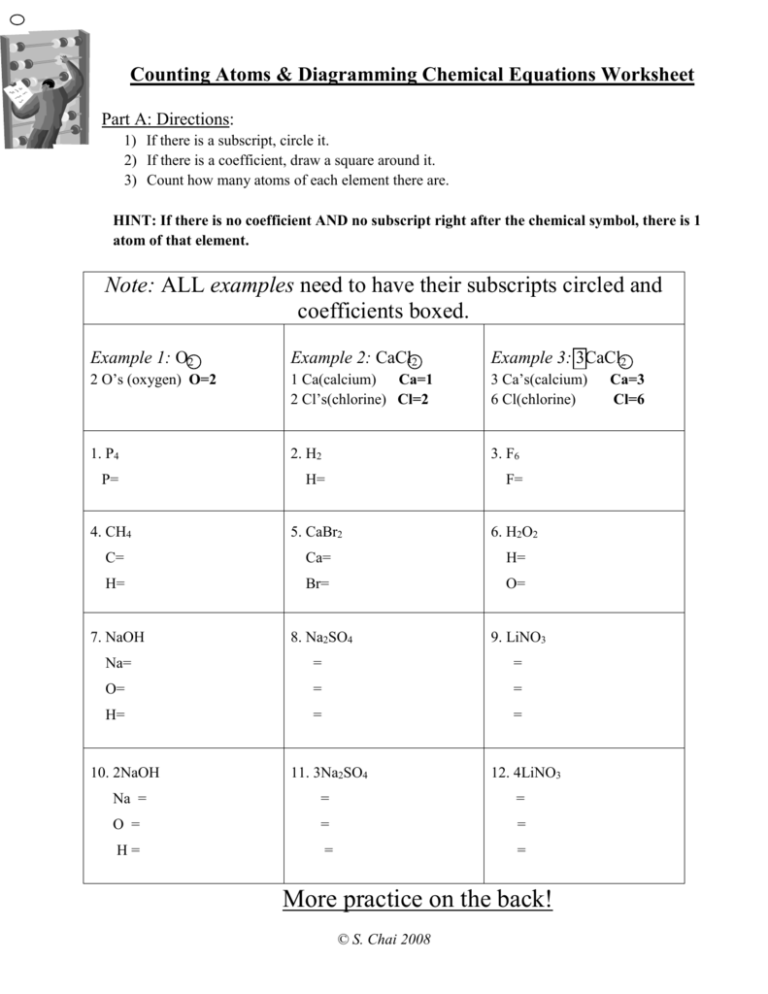
Counting Atoms & Diagramming Chemical Equations Worksheet Part A: Directions: 1) If there is a subscript, circle it. 2) If there is a coefficient, draw a square around it. 3) Count how many atoms of each element there are. HINT: If there is no coefficient AND no subscript right after the chemical symbol, there is 1 atom of that element. Note: ALL examples need to have their subscripts circled and coefficients boxed. Example 1: O2 Example 2: CaCl2 Example 3: 3CaCl2 2 O’s (oxygen) O=2 1 Ca(calcium) Ca=1 2 Cl’s(chlorine) Cl=2 3 Ca’s(calcium) 6 Cl(chlorine) 1. P4 2. H2 3. F6 P= H= F= 4. CH4 5. CaBr2 6. H2O2 C= Ca= H= H= Br= O= 7. NaOH 8. Na2SO4 9. LiNO3 Na= = = O= = = H= = = 10. 2NaOH 11. 3Na2SO4 12. 4LiNO3 Na = = = O = = = H= = = More practice on the back! © S. Chai 2008 Ca=3 Cl=6 Part B: Directions: 1) Diagram the following equations by: a. circling the reactants in the equation b. Putting a square around the products in the reaction 2) Count the # of atoms on each side and write it down. 3) Answer if the equation is balanced or not by circling yes or no. Example 4: Example 5: HgO → Hg + O2 Products CH3 + 2O2 Products C= 1 Reactants Hg = 1 Hg = 1 O =1 O =2 Is this equation balanced? Yes / No Reactants C=1 H= 3 H=4 O= 4 O=4 Is this equation balanced? Yes / No 13. P + O2 → P4O10 Products CO2 + 2H2O 14. Mg + O2 → MgO Reactants Products Reactants P= P= Mg = Mg = O= O= O= O= Is this equation balanced? Yes / No Is this equation balanced? Yes / No 15. 4 Fe = 3O2 → 2 Fe2O3 16. Al2O3 → Al + O2 Products Products Reactants Reactants Fe = Fe = Al = Al = O= O= O= O= Is this equation balanced? Yes / No Is this equation balanced? Yes / No CHALLENGE (#17) BaCl2 + H2SO4 → BaSO4 + HCl Products ___ Reactants Is this equation balanced? Yes / No © S. Chai 2008











